Melatonin as a promising agent alleviating endocrine deregulation and concurrent cardiovascular dysfunction: a review and future prospect
Melatonin in hormone-induced cardiovascular impairment
Abstract
Endocrine modulation of various growth and survival mechanisms is at the helm of cellular homeostasis and impaired endocrine balance may potentially galvanize cardiovascular health to go haywire. Melatonin, an effective antioxidant and multipotent hormone has preponderant influence on the activities of several endocrine factors including growth hormones, thyroid hormones, gastro-intestinal hormones, and those controlling reproductive and metabolic functions. Many of these hormones tightly regulate cardiovascular functions while the mammalian heart has its own endocrine machinery. Endocrine disruptions severely affect cardiovascular integrity and hormonal therapies may instigate adverse cardiac events. Therefore, this review focuses on the cardioprotective potential of melatonin concerning endocrine instability-mediated cardiovascular dysfunction. Melatonin has been reported to effectively counteract sympathetic overstimulation and also reduce the cardiotoxic attributes of catecholamines and their derivatives. Melatonin suppresses the pernicious cardiovascular manifestation of thyrotoxicosis and autoimmune thyroiditis, which is possibly attributed to its antioxidant property and regulation of iodothyronine-deiodinase activity. Interestingly, being a circadian synchronizer melatonin potentially preserves the diurnal pattern of insulin secretion and thereby improves glucose tolerance and cardiac GLUT-4 expression. Besides, melatonin modulates insulin signaling pathway by enhancing the activation of insulin receptor-associated tyrosine kinase, thus protecting the heart against diabetogenic outcomes. Further, melatonin has demonstrated its beneficial action against non-dipper hypertension by regulating the RAAS function. However, there is a plethora of unresolved research question that necessitates additional investigation into the potential therapeutic effect of melatonin in endocrine dysfunctions that emanates during various physiological and pathological states and may have potentially harmful cardiovascular implications.
References
2. Ibarra C, Lavandero S, Estrada M (2015) Regulation of cardiovascular metabolism by hormones and growth factors. Int. J. Endocrinol. 2015: 842351. DOI: 10.1155/2015/842351.
3. Ogawa T, de Bold AJ (2014) The heart as an endocrine organ. Endocr. Connect. 3: R31-R44. DOI: 10.1530/EC-14-0012.
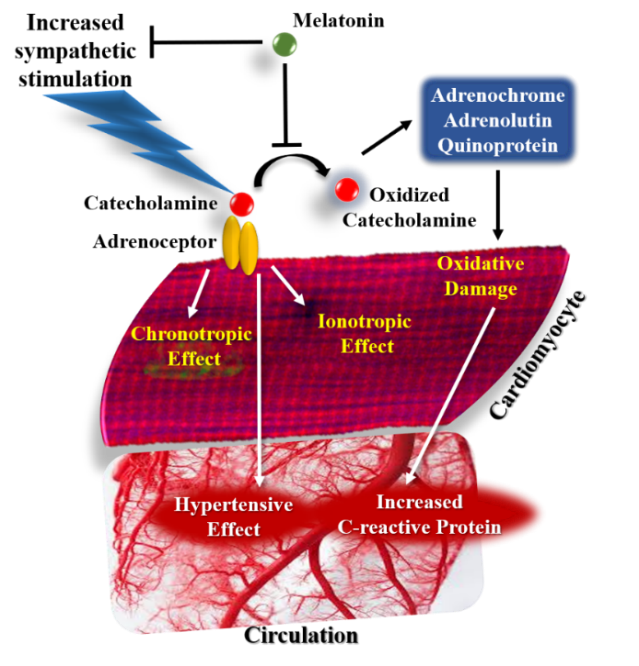
4. Hall ME, Yanes L, Long RC, Koch CA (2015) Hormones of the cardiovascular system. Endotext [Internet].
5. Kolwicz SC Jr, Purohit S, Tian R (2013) Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ. Res. 113: 603-616. DOI:10.1161/CIRCRESAHA.113.302095.
6. McGiff JC (1968) Tissue hormones: angiotensin, bradykinin and the regulation of regional blood flows. Med. Clin. N. 52: 263-281.
7. Moolman JA (2006) Unravelling the cardioprotective mechanism of action of estrogens Cardiovasc. Res. 69: 777–780. DOI: https://doi.org/10.1016/j.cardiores.2006.01.001.
8. Burley DS, Hamid SA, Baxter GF (2007) Cardioprotective actions of peptide hormones in myocardial ischemia. Heart Fail. Rev. 12: 279. DOI: https://doi.org/10.1007/s10741-007-9029-y
9. Reiter RJ, Calvo JR, Karbownik M, Qi W, Tan DX (2000) Melatonin and its relation to the immune system and inflammation. Ann. N. Y. Acad. Sci. 917: 376-386. DOI: 10.1111/j.1749-6632.2000.tb05402.x.
10. Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra MC, Hardeland R (2002) Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2: 181-197. DOI: 10.2174/1568026023394443.
11. Dominguez-Rodriguez A, Abreu-Gonzalez P, Chen Y (2019) Cardioprotection and effects of melatonin administration on cardiac ischemia reperfusion: Insight from clinical studies. Melatonin Res. 2: 100-105. DOI: https://doi.org/https://doi.org/10.32794/mr11250024.
12. Sarkar S, Chattopadhyay A, Bandyopadhyay D (2021) Melatonin as a prospective metabolic regulator in pathologically altered cardiac energy homeostasis. Melatonin Res. 4: 316-335. DOI: https://doi.org/10.32794/mr11250097.
13. Sarkar S, Chattopadhyay A, Bandyopadhyay D (2020) Melatonin, the advance-guard in oxidative myocardial assault instigated by exercise stress: a physiological and biochemical insight. Melatonin Res. 3: 451-475. DOI: https://doi.org/https://doi.org/10.32794/mr11250072.
14. Cardinali DP (1981) Melatonin. A mammalian pineal hormone. Endocr Rev. 2: 327-346. DOI: 10.1210/edrv-2-3-327.
15. Pandi-Perumal SR, BaHammam AS, Ojike NI, Akinseye OA, Kendzerska T, Buttoo K, Dhandapany PS, Brown GM, Cardinali DP (2017) Melatonin and human cardiovascular disease. J. Cardiovasc. Pharmacol. Ther. 22: 122-132. DOI:10.1177/1074248416660622.
16. Sarkar S, Chattopadhyay A, Bandyopadhyay D (2021) Multiple strategies of melatonin protecting against cardiovascular injury related to inflammation: A comprehensive overview. Melatonin Res. 4: 1-29. DOI: 10.32794/mr11250080.
17. Carrillo‐Vico A, Lardone PJ, Naji L, Fernández‐Santos JM, Martín‐Lacave I, Guerrero JM, Calvo JR (2005) Beneficial pleiotropic actions of melatonin in an experimental model of septic shock in mice: regulation of pro-/anti-inflammatory cytokine network, protection against oxidative damage and anti-apoptotic effects. J. Pineal Res. 39: 400-408. DOI: 10.1111/j.1600-079X.2005.00265.x.
18. Jiki Z, Lecour S, Nduhirabandi F (2018) Cardiovascular benefits of dietary melatonin: a myth or a reality? Front. Physiol. 9: 528. DOI: 10.3389/fphys.2018.00528.
19. Deegan RJ, Furman WR (2011) Cardiovascular manifestations of endocrine dysfunction. J. Cardiothorac. Vasc. Anesth. 25: 705-720. DOI: 10.1053/j.jvca.2010.12.001.
20. Binu AJ, Cherian KE, Kapoor N, Chacko ST, George O, Paul TV (2017) The Heart of the Matter: Cardiac Manifestations of Endocrine Disease. Indian J. Endocrinol. Metab. 21: 919-925. DOI: 10.4103/ijem.IJEM_212_17.
21. Cipolla-Neto J, Amaral FGD (2018) Melatonin as a hormone: New physiological and clinical insights. Endocr Rev. 39: 990-1028. DOI: 10.1210/er.2018-00084.
22. McCord CP, Allen FP (1917) Evidences associating pineal gland function with alterations in pigmentation. Exp. Zool. 23: 207-224. DOI: 10.1002/jez.1400230108.
23. Lerner AB, Case JD, Heinzelman RY (1959) Structure of melatonin. Am. Chem. Soc. 81: 6084-6087.
24. Axelrod J, Weisbach H (1961) Purification and properties of hydroxyindole-O-methyltransferase. Biol. Chem. 236: 211-213. DOI: 10.1016/S0021-9258(18)64458-8.
25. Wurtman RJ, Axelrod J, Chu W (1963) Melatonin, a pineal substance: effect on rat ovary. Science 141: 277-278. DOI: 10.1126/science.141.3577.277.
26. Tan DX, Manchester LC, Hardeland R, Lopez-Burillo S, Mayo JC, Sainz RM, Reiter RJ (2003) Melatonin: a hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J. Pineal Res. 34: 75-78. DOI: 10.1034/j.1600-079x.2003.02111.x.
27. Jockers R, Delagrange P, Dubocovich ML, Markus RP, Renault N, Tosini G, Cecon E, Zlotos DP (2016) Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol. 173: 2702–2725. DOI: 10.1111/bph.13536.
28. Voiculescu SE, Zygouropoulos N, Zahiu CD, Zagrean AM (2014) Role of melatonin in embryo fetal development. J. Med. Life. 7: 488-492.
29. Korkmaz A, Topal T, Tan DX, Reiter RJ (2009) Role of melatonin in metabolic regulation. Rev. Endocr. Metab. Disord. 10: 261-270. DOI: 10.1007/s11154-009-9117-5.
30. Zisapel N (2018) New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 175: 3190-3199. DOI: 10.1111/bph.14116.
31. Reiter RJ, Tan DX, Manchester LC, Paredes SD, Mayo JC, Sainz RM (2009) Melatonin and reproduction revisited. Biol. Reprod. 81: 445-456. DOI: 10.1095/biolreprod.108.075655.
32. Utiger RD (1992) Melatonin--the hormone of darkness. N. Engl. J. Med. 327: 1377-1379. DOI: 10.1056/NEJM199211053271909.
33. Lynch HJ, Wurtman RJ, Moskowitz MA, Archer MC, Ho MH (1975) Daily rhythm in human urinary melatonin. Science 187:169-171. DOI: 10.1126/science.1167425.
34. Czeisler CA, Shanahan TL, Klerman EB, Martens H, Brotman DJ, Emens JS, Klein T, Rizzo JF 3rd (1995) Suppression of melatonin secretion in some blind patients by exposure to bright light. N. Engl. J. Med. 332: 6-11. DOI: 10.1056/NEJM199501053320102.
35. Kennaway DJ, Stamp GE, Goble FE (1992) Development of melatonin production in infants and the impact of prematurity. Clin. Endocrinol.Metab. 75: 367-369. DOI: 10.1210/jcem.75.2.1639937.
36. Zhdanova IV, Lynch HJ, Wurtman RJ (1997) Melatonin: a sleep-promoting hormone. Sleep 20: 899-907.
37. Waldhauser F, Weiszenbacher G, Tatzer E, Gisinger B, Waldhauser M, Schemper M, Frisch H (1988) Alterations innocturnal serum melatonin levels in humans with growth andaging. Clin. Endocrinol. Metab. 66: 648-652. DOI: 10.1210/jcem-66-3-648.
38. Hardeland R, Balzer I, Poeggeler B, Fuhrberg B, Uría H, Behrmann G, Wolf R, Meyer TJ, Reiter RJ (1995) On the primaryfunctions of melatonin in evolution: mediation of photoperiodic signals in a unicell, photooxidation, and scavenging offree radicals. J. Pineal Res. 18: 104–111. DOI: 10.1111/j.1600-079x.1995.tb00147.x.
39. Tilden AR, Becker MA, Amma LL, Arciniega J, McGaw AK (1997) Melatonin production in an aerobic photosynthetic bacterium: an evolutionarily early association with darkness. J. Pineal Res. 22: 102-106. DOI: 10.1111/j.1600-079x.1997.tb00310.x.
40. Venegas C, García JA, Escames G, Ortiz F, López A, Doerrier C, García-Corzo L, López LC, Reiter RJ, Acuña-Castroviejo D (2012) Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 52: 217-227. DOI: 10.1111/j.1600-079X.2011.00931.x.
41. Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX, Reiter RJ (2014) Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol. Life Sci. 71 (16): 2997-3025. DOI: 10.1007/s00018-014-1579-2.
42. de Almeida EA, Di Mascio P, Harumi T, Spence DW, Moscovitch A, Hardeland R, Cardinali DP, Brown GM, Pandi-Perumal SR (2011) Measurement of melatonin in body fluids: standards, protocols and procedures. Childs Nerv. Syst. 27: 879-891. DOI: 10.1007/s00381-010-1278-8.
43. Grundy SM (2004) Obesity, metabolic syndrome, and cardiovascular disease. J. Clin. Endocrinol. Metab. 89: 2595-2600. DOI: 10.1210/jc.2004-0372.
44. Bruce KD, Byrne CD (2009) The metabolic syndrome: common origins of a multifactorial disorder. Postgrad. Med. J. 85: 614-621. DOI: 10.1136/pgmj.2008.078014.
45. Nikolopoulou, A, Kadoglou NP (2012) Obesity and metabolic syndrome as related to cardiovascular disease. Expert Rev. Cardiovasc. Ther. 10: 933-939. DOI: 10.1586/erc.12.74.
46. Zeman M, Buyse J, Lamosová D, Herichová I, Decuypere E (1999) Role of melatonin in the control of growth and growth hormone secretion in poultry. Domest. Anim. Endocrinol.17: 199-207. DOI: 10.1016/s0739-7240(99)00037-5.
47. Reiter RJ (1973) Comparative physiology: pineal gland. Annu. Rev. Physiol. 35: 305-328. DOI: 10.1146/annurev.ph.35.030173.001513.
48. Reiter RJ (1980) The pineal and its hormones in the control of reproduction in mammals. Endocr. Rev.1: 109-131. DOI: 10.1210/edrv-1-2-109.
49. Reiter RJ, Hurlbut EC, Brainard GC, Steinlechner S, Richardson BA (1983) Influence of light irradiance on hydroxyindole-O-methyltransferase activity, serotonin-N-acetyltranferase activity, and radioimmunoassayable melatonin levels in the pineal gland of the diurnally active Richardson's ground squirrel. Brain Res. 288: 151-157. DOI: 10.1016/0006-8993(83)90089-6.
50. Smythe GA, Lazarus L (1973) Growth hormone regulation by melatonin and serotonin. Nature 244: 230-231. DOI: 10.1038/244230a0.
51. Lamosˇova´ D, Jura´ni M, Vanekova´ D (1994) In vitro separation of embryonic chick skeletal muscle myoblasts and fibroblasts: comparison of their characteristics. Physiol. Res. 43: 157–161.
52. Lamosˇova´ D, Zeman M, Jura´ni M (1997) Influence of melatonin on chick skeletal muscle cell growth. Comp. Biochem. Physiol. 118C: 375–379. DOI: 10.1016/s0742-8413(97)00159-x.
53. Harvey S, Fraser RA, Lea RW (1991) Growth hormone secretion in poultry. Crit. Rev. Poult. Biol. 3: 239–282.
54. Harvey S, Hall TR (1987) Somatostatin immunoneutralization overcomes the inhibitory effects of quipazine and pargyline on growth hormone secretion in domestic fowl. Experientia 43: 602–604. DOI: 10.1007/BF02126345.
55. Klocek-Gorka B, Szczesna M, Molik E, Zieba DA (2010) The interactions of season, leptin and melatonin levels with thyroid hormone secretion, using an in vitro approach. Small Rumin. Res. 91: 231–235.
56. Quignon C, Beymer M, Gauthier K, Gauer F, Simonneaux V (2020) Thyroid hormone receptors are required for the melatonin-dependent control of Rfrp gene expression in mice. FASEB J. 34: 12072-12082. DOI: 10.1096/fj.202000961R.
57. Taheri P, Mogheiseh A, Shojaee Tabrizi A, Nazifi S, Salavati S, Koohi F (2019) Changes in thyroid hormones, leptin, ghrelin, and galanin following oral melatonin administration in intact and castrated dogs: a preliminary study. BMC Vet. Res. 15: 1-13. DOI: 10.1186/s12917-019-1894-9.
58. Kirsz K, Szczęsna M, Borsuk A, Zięba DA (2017) Cross-talk between leptin, ghrelin and orexins in the central nervous system of seasonal animals – a review. Ann. Anim. Sci. 17: 337–350. DOI: 10.1515/aoas-2016-0070.
59. Juszczak M, Bojanowska E, Dabrowski R (2000) Melatonin and the synthesis of vasopressin in pinealectomized male rats. Proc. Soc. Exp. Biol. Med. 225: 207–210. DOI: https://doi.org/10.1177/153537020022500307.
60. Rintamaki H, Hissa R, Balthazart J, Scanes CG (1984) The effect of pinealectomy on plasma levels of gonadotrophins and growth hormone in the pigeon (Columba livia). J. Pineal Res. 1: 381–389. DOI: 10.1111/j.1600-079x.1984.tb00228.x.
61. Cahill DJ, Wardle PG, Harlow CR, Hull MG (1998) Onset of the preovulatory luteinizing hormone surge: diurnal timing and critical follicular prerequisites. Fertil. Steril. 70: 56–59. DOI: 10.1016/S0015-0282 (98)00113-7.
62. Russo KA, La JL, Stephens SB, Poling MC, Padgaonkar NA (2015) Circadian control of the female reproductive axis through gated responsiveness of the RFRP-3 system to VIP signaling. Endocrinology 156: 2608–2618. DOI: 10.1210/en.2014-1762.
63. Brzezinski A, Seibel MM, Lynch HJ, Deng MH, Wurtman RJ (1987) Melatonin in human preovulatory follicular fluid. J. Clin. Endocrinol. Metab. 64: 865–867. DOI: 10.1210/jcem-64-4-865.
64. Olcese JM (2020) Melatonin and female reproduction: an expanding universe. Front. Endocrinol. (Lausanne).11: 85. DOI: 10.3389/fendo.2020.00085.
65. Scarinci E, Tropea A, Notaristefano G, Arena V, Alesiani O, Fabozzi SM, Lanzone A, Apa R (2019) "Hormone of darkness" and human reproductive process: direct regulatory role of melatonin in human corpus luteum. J. Endocrinol. Invest. 42: 1191-1197. DOI: 10.1007/s40618-019-01036-3.
66. Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L, et al. (2012) The role of melatonin as an antioxidant in the follicle. J. Ovarian Res. 5: 5. DOI: 10.1186/1757-2215-5-5.
67. Sharkey JT, Puttaramu R, Word RA, Olcese J (2009) Melatonin synergizes with oxytocin to enhance contractility of human myometrial smooth muscle cells. J. Clin. Endocrinol. Metab. 94: 421-427. DOI: 10.1210/jc.2008-1723.
68. Jung UJ, Choi MS (2014) Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 15: 6184-6223. DOI: 10.3390/ijms15046184.
69. Lage R, Diéguez C, Vidal-Puig A, López M (2008) AMPK: a metabolic gauge regulating whole-body energy homeostasis. Trends Mol. Med. 14: 539-549. DOI: 10.1016/j.molmed.2008.09.007.
70. Bruch C, Herrmann B, Schmermund A, Bartel T, Mann K, Erbel R (2002) Impact of disease activity on left ventricular performance in patients with acromegaly. Am. Heart J. 144: 538-543. DOI: 10.1067/mhj.2002.123572.
71. Damjanovic SS, Neskovic AN, Petakov MS, Popovic V, Vujisic B, Petrovic M, Nikolic-Djurovic M, Simic M, Pekic S, Marinkovic J (2002) High output heart failure in patients with newly diagnosed acromegaly. Am. J. Med. 112: 610-616. DOI: 10.1016/s0002-9343(02)01094-x.
72. Melmed S (2006) Medical progress: Acromegaly. N. Engl. J. Med. 355: 2558-2573. doi: 10.1056/NEJMra062453.
73. Maison P, Tropeano AI, Macquin-Mavier I, Giustina A, Chanson P (2007) Impact of somatostatin analogs on the heart in acromegaly: a metaanalysis. J. Clin. Endocrinol. Metab. 92: 1743-1747. DOI: 10.1210/jc.2006-2547.
74. Ong SL, Whitworth JA (2011) How do glucocorticoids cause hypertension: role of nitric oxide deficiency, oxidative stress, and eicosanoids. Endocrinol. Metab. Clin. North Am. 40: 393-407. DOI: 10.1016/j.ecl.2011.01.010.
75. Byatt JC, Staten NR, Salsgiver WJ, Kostelc JG, Collier RJ (1993) Stimulation of food intake and weight gain in mature female rats by bovine prolactin and bovine growth hormone. Am. J. Physiol. 264: E986-E992. DOI: 10.1152/ajpendo.1993.264.6.E986.
76. Haring R, Friedrich N, Völzke H, Vasan RS, Felix SB, Dörr M, Meyer zu Schwabedissen HE, Nauck M, Wallaschofski H (2014) Positive association of serum prolactin concentrations with all-cause and cardiovascular mortality. Eur. Heart J. 35: 1215-1221. DOI: 10.1093/eurheartj/ehs233.
77. Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, Luchtefeld M, Poli V, Schneider MD, Balligand JL, Desjardins F, Ansari A, Struman I, Nguyen NQ, Zschemisch NH, Klein G, Heusch G, Schulz R, Hilfiker A, Drexler H (2007) A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 128: 589-600. DOI: 10.1016/j.cell.2006.12.036.
78. Nishizawa S, Nakamura T, Hamaoka T, Matsumuro A, Sawada T, Matsubara H (2009) Lethal arrhythmia and corticosteroid insufficiency. Am. J. Emerg. Med. 27: 1167.e1-3. DOI: 10.1016/j.ajem.2008.12.001.
79. Lombardi G, Di Somma C, Grasso LF, Savanelli MC, Colao A, Pivonello R (2012) The cardiovascular system in growth hormone excess and growth hormone deficiency. J. Endocrinol. Invest. 35: 1021-1029. doi: 10.3275/8717.
80. Hu ZP, Fang XL, Fang N, Wang XB, Qian HY, Cao Z, Cheng Y, Wang BN, Wang Y (2013) Melatonin ameliorates vascular endothelial dysfunction, inflammation, and atherosclerosis by suppressing the TLR 4/NF-κB system in high‐fat‐fed rabbits. J. Pineal Res. 55: 388-398. DOI: 10.1111/jpi.12085.
81. Yu L, Liang H, Lu Z, Zhao G, Zhai M, Yang Y, Yang J, Yi D, Chen W, Wang X, Duan W (2015) Membrane receptor‐dependent Notch1/Hes1 activation by melatonin protects against myocardial ischemia–reperfusion injury: in vivo and in vitro studies. J. Pineal Res. 59: 420-433. DOI: 10.1111/jpi.12272.
82. Reiter RJ, Sharma R, Chuffa LGdA, Simko F, Dominguez-Rodriguez A (2024) Mitochondrial melatonin: beneficial effects in protecting against heart failure. Life 14: 88. DOI: https://doi.org/10.3390/life14010088.
83. Thonusin C, Nawara W, Arinno A, Khuanjing T, Prathumsup N, Ongnok B, Chattipakorn SC, Chattipakorn N (2023) Effects of melatonin on cardiac metabolic reprogramming in doxorubicin-induced heart failure rats: A metabolomics study for potential therapeutic targets. J. Pineal. Res. 75: e12884. DOI: 10.1111/jpi.12884.
84. Dominguez-Rodriguez A, Abreu-Gonzalez P, Reiter R J (2014) The potential usefulness of serum melatonin level to predict heart failure in patients with hypertensive cardiomyopathy. Int. J. Cardiol. 174: 415-417. DOI: 10.1016/j.ijcard.2014.04.044.
85. Vischer UM, Wollheim CB (1997) Epinephrine induces von Willebrand factor release from cultured endothelial cells: involvement of cyclic AMP-dependent signalling in exocytosis. Thromb. Haemost. 77: 1182-1188.
86. Attaran RR, Ewy GA (2010) Epinephrine in resuscitation: curse or cure? Future Cardiol. 6: 473-482. DOI: 10.2217/fca.10.24.
87. Thomas SR, Chen K, Keaney JF Jr (2002) Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J. Biol. Chem. 277: 6017-6024. DOI: 10.1074/jbc.M109107200.
88. Schade R, Göhler K, Bürger W, Hirschelmann R (1987) Modulation of rat C-reactive protein serum level by dexamethasone and adrenaline--comparison with the response of alpha 2-acute phase globulin. Agents Actions. 22: 280-287. DOI: 10.1007/BF02009057.
89. Aninat C, Seguin P, Descheemaeker PN, Morel F, Malledant Y, Guillouzo A (2008) Catecholamines induce an inflammatory response in human hepatocytes. Crit. Care Med. 36 (3): 848-54. doi: 10.1097/CCM.0B013E31816532BE.
90. Costa VM, Silva R, Ferreira LM, Branco PS, Carvalho F, Bastos ML, Carvalho RA, Carvalho M, Remião F (2007) Oxidation process of adrenaline in freshly isolated rat cardiomyocytes: formation of adrenochrome, quinoproteins, and GSH adduct. Chem. Res. Toxicol. 20: 1183-1191. DOI:10.1021/tx7000916.
91. Freire F, Cardinali DP (1975) Effects of melatonin treatment and environmental lighting on the ultrastructural appearence, melatonin synthesis, norepinephrine turnover and microtubule protein content of the rat pineal gland. J. Neural Transm. 37: 237–257. DOI: 10.1007/BF01670132.
92. Viswanathan M, Hissa R, George JC (1986) Suppression of sympathetic nervous system by short photoperiod and melatonin in the Syrian hamster. Life Sci. 38: 73-79. DOI: 10.1016/0024-3205(86)90277-8.
93. Paulis L, Simko F (2007) Blood pressure modulation and cardiovascular protection by melatonin: potential mechanisms behind. Physiol. Res. 56: 671-684. DOI: 10.33549/physiolres.931236.
94. Girouard H, Chulak C, LeJossec M, Lamontagne D, de Champlain J (2003) Chronic antioxidant treatment improves sympathetic functions and beta-adrenergic pathway in the spontaneously hypertensive rats. J. Hypertens. 21: 179-188. DOI: 10.1097/00004872-200301000-00028.
95. Girouard H, Denault C, Chulak C, De Champlain J (2004) Treatment by N-acetylcysteine and melatonin increases cardiac baroreflex and improves antioxidant reserve. Am. J. Hypertens. 17: 947-954. DOI: 10.1016/j.amjhyper.2004.06.009.
96. Repova K, Aziriova S, Krajcirovicova K, Simko F (2022) Cardiovascular therapeutics: A new potential for anxiety treatment? Med. Res. Rev. 42: 1202-1245. DOI: 10.1002/med.21875.
97. Repova K, Baka T, Krajcirovicova K, Stanko P, Aziriova S, Reiter RJ, Simko F (2022) Melatonin as a potential approach to anxiety treatment. Int. J. Mol. Sci. 23: 16187. DOI: 10.3390/ijms232416187.
98. Klein I, Ojamaa K (2001) Thyroid hormone and the cardiovascular system. N. Engl. J. Med. 344: 501-509. DOI:10.1056/NEJM200102153440707.
99. Cooper DS, Biondi B (2012) Subclinical thyroid disease. Lancet 379: 1142-1154. DOI:10.1016/S0140-6736(11)60276-6
100. Mestman JH (2004) Hyperthyroidism in pregnancy. Best Pract. Res. Clin. Endocrinol. Metab. 18: 267-288. DOI:10.1016/j.beem.2004.03.005.
101. Sanyal D, Ray S, Singh MH (2022) Interplay between cardiovascular and thyroid dysfunctions: A review of clinical implications and management strategies. Endocr. Regul. 56: 311-328.DOI: 10.2478/enr-2022-0033.
102. Kahaly GJ, Dillmann WH (2005) Thyroid hormone action in the heart. Endocr. Rev. 26: 704–728. DOI: https://doi.org/10.1210/er.2003-0033.
103. Fernández-Santos JM, Morillo-Bernal J, García-Marín R, Utrilla JC, Martín-Lacave I (2012) Paracrine regulation of thyroid-hormone synthesis by C cells. Thyroid hormone. DOI: 10.5772/46178.
104. Lewinski A, Karbownik M (2002) REVIEW. Melatonin and the thyroid gland. Neuro. Endocrinol. Lett. 23 Suppl 1: 73-78.
105. D'Angelo G, Marseglia L, Manti S, Colavita L, Cuppari C, Impellizzeri P, Arena S, Arrigo T, Salpietro C, Gitto E (2016) Atopy and autoimmune thyroid diseases: melatonin can be useful? Ital. J. Pediatr. 42: 95. DOI:10.1186/s13052-016-0305-0.
106. Xiang L, Mittwede PN, Clemmer JS (2015) Glucose homeostasis and cardiovascular alterations in diabetes. Compr. Physiol. 5: 1815-1839. DOI:10.1002/cphy.c150001.
107. Wondmkun YT (2020) Obesity, insulin resistance, and type 2 diabetes: Associations and therapeutic implications. Diabetes Metab. Syndr. Obes. 13: 3611–3616. https://doi.org/10.2147/DMSO.S275898.
108. Kido Y, Nakae J, Accili D (2001) Clinical review 125: The insulin receptor and its cellular targets. J. Clin. Endocrinol. Metab. 86: 972-979. DOI:10.1210/jcem.86.3.7306.
109. Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 329: 977-986. DOI:10.1056/NEJM199309303291401.
110. Kahn SE, Cooper ME, Del Prato S (2014) Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 383: 1068-1083. DOI:10.1016/S0140-6736(13)62154-6.
111. Khullar M, Al-Shudiefat AA, Ludke A, Binepal G, Singal PK (2010) Oxidative stress: A key contributor to diabetic cardiomyopathy. Can. J. Physiol. Pharmacol. 88: 233-240. DOI:10.1139/Y10-016.
112. la Fleur SE, Kalsbeek A, Wortel J, van der Vliet J, Buijs RM (2001) Role for the pineal and melatonin in glucose homeostasis: pinealectomy increases night-time glucose concentrations. J. Neuroendocrinol. 13: 1025-1032. DOI:10.1046/j.1365-2826.2001.00717.x.
113. Picinato MC, Hirata AE, Cipolla-Neto J, Curi R, Carvalho CR, Anhê GF, Carpinelli AR. (2008) Activation of insulin and IGF-1 signaling pathways by melatonin through MT1 receptor in isolated rat pancreatic islets. J. Pineal Res. 44: 88-94. DOI: 10.1111/j.1600-079X.2007.00493.x.
114. Brydon L, Petit L, Delagrange P, Strosberg AD, Jockers R (2001) Functional expression of MT2 (Mel1b) melatonin receptors in human PAZ6 adipocytes. Endocrinology 142: 4264-4271. DOI:10.1210/endo.142.10.8423.
115. Dragoi CM, Arsene AL, Dinu-Pirvu CE, Dumitrescu IB, Popa D, Burcea-Dragomiroiu GTA, Nicolae AC (2017) Melatonin: A silent regulator of the glucose homeostasis. InTech. DOI: 10.5772/66625.
116. Mühlbauer E, Peschke E (2007) Evidence for the expression of both the MT1- and in addition, the MT2-melatonin receptor, in the rat pancreas, islet and beta-cell. J. Pineal Res. 42: 105-106. DOI:10.1111/j.1600-079X.2006.00399.x.
117. Sharma S, Singh H, Ahmad N, Mishra P, Tiwari A (2015) The role of melatonin in diabetes: therapeutic implications. Arch. Endocrinol. Metab. 59: 391-399. DOI:10.1590/2359-3997000000098.
118. Peschke E, Bahr I, Muhlbauer E (2013) Melatonin and pancreatic islets: Interrelationships between melatonin, insulin and glucagon. Int. J. Mol. Sci. 14: 6981-7015.DOI:10.3390/ijms14046981.
119. Owino S, Buonfiglio DDC, Tchio C, Tosini G (2019) Melatonin signaling a key regulator of glucose homeostasis and energy metabolism. Front. Endocrinol. (Lausanne). 10: 488. DOI:10.3389/fendo.2019.00488.
120. Sadoshima J, Izumo S (1993) Molecular characterization of angiotensin II-induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ. Res. 73: 413-423. DOI:10.1161/01.res.73.3.413.
121. Baruch L, Anand I, Cohen IS, Ziesche S, Judd D, Cohn JN (1999) Augmented short- and long-term hemodynamic and hormonal effects of an angiotensin receptor blocker added to angiotensin converting enzyme inhibitor therapy in patients with heart failure. Circulation 99: 2658-2664. DOI:10.1161/01.cir.99.20.2658.
122. Rodrigues EJ, Eisenberg MJ, Pilote L (2003) Effects of early and late administration of angiotensin-converting enzyme inhibitors on mortality after myocardial infarction. Am. J. Med. 115: 473-479. DOI:10.1016/s0002-9343(03)00435-2.
123. Doulton TW, He FJ, MacGregor GA (2005) Systematic review of combined angiotensin-converting enzyme inhibition and angiotensin receptor blockade in hypertension. Hypertension 45: 880-886. DOI:10.1161/01.HYP.0000161880.59963.da.
124. Ma TK, Kam KK, Yan BP, Lam YY (2010) Renin-angiotensin-aldosterone system blockade for cardiovascular diseases: current status. Br. J. Pharmacol. 160: 1273-1292. DOI:10.1111/j.1476-5381.2010.00750.x.
125. Meng W, Zhao W, Zhao T, Liu C, Chen Y, Liu H, Sun Y (2014) Autocrine and paracrine function of Angiotensin 1-7 in tissue repair during hypertension. Am. J. Hypertens. 27: 775-782. DOI:10.1093/ajh/hpt270.
126. Baltatu O, Nishimura H, Hoffmann S, et al. (1997) High levels of human chymase expression in the pineal and pituitary glands. Brain Res. 752: 269-278. DOI:10.1016/s0006-8993(96)01474-6
127. Campos LA, Cipolla-Neto J, Amaral FG, Michelini LC, Bader M, Baltatu OC (2013) The Angiotensin-melatonin axis. Int. J. Hypertens. 2013: 521783. DOI:10.1155/2013/521783.
128. Lemmer B, Witte K, Enzminger H, Schiffer S, Hauptfleisch S (2003) Transgenic TGR (mREN2)27 rats as a model for disturbed circadian organization at the level of the brain, the heart, and the kidneys. Chronobiol. Int. 20: 711-738. DOI:10.1081/cbi-120022407.
129. Hermida RC, Ayala DE, Mojón A, Fernández JR (2013) Blunted sleep-time relative blood pressure decline increases cardiovascular risk independent of blood pressure level--the "normotensive non-dipper" paradox. Chronobiol. Int. 30: 87-98. DOI: 10.3109/07420528.2012.701127.
130. Duez H, Staels B (2010) Nuclear receptors linking circadian rhythms and cardiometabolic control. Arterioscler. Thromb. Vasc. Biol. 30: 1529-1534.https://doi.org/10.1161/ATVBAHA.110.209098.
131. Ménard J, Rigel DF, Watson C, Jeng AY, Fu F, Beil M, Liu J, Chen W, Hu CW, Leung-Chu J, LaSala D, Liang G, Rebello S, Zhang Y, Dole WP (2014) Aldosterone synthase inhibition: cardiorenal protection in animal disease models and translation of hormonal effects to human subjects. J. Transl. Med. 12: 340. DOI: https://doi.org/10.1186/s12967-014-0340-9.
132. Jonas M, Garfinkel D, Zisapel N, Laudon M, Grossman E (2003) Impaired nocturnal melatonin secretion in non-dipper hypertensive patients. Blood Press. 12: 19–24.
133. Douma LG, Gumz ML (2018) Circadian clock-mediated regulation of blood pressure. Free Radic. Biol. Med. 119: 108–114. DOI: https://doi.org/10.1016/j.freeradbiomed.2017.11.024.
134. Lee EK, Poon P, Yu CP, Lee VW, Chung VC, Wong SY (2022) Controlled-release oral melatonin supplementation for hypertension and nocturnal hypertension: A systematic review and meta-analysis. J. Clin. Hypertens. (Greenwich, Conn.). 24: 529–535. DOI: https://doi.org/10.1111/jch.14482.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


