Melatonin as a protective adjunct to the renin angiotensin system imbalance induced cardiovascular pathogenesis: A review
Melatonin protects from Renin Angiotensin system induced cardiovascular injury
Abstract
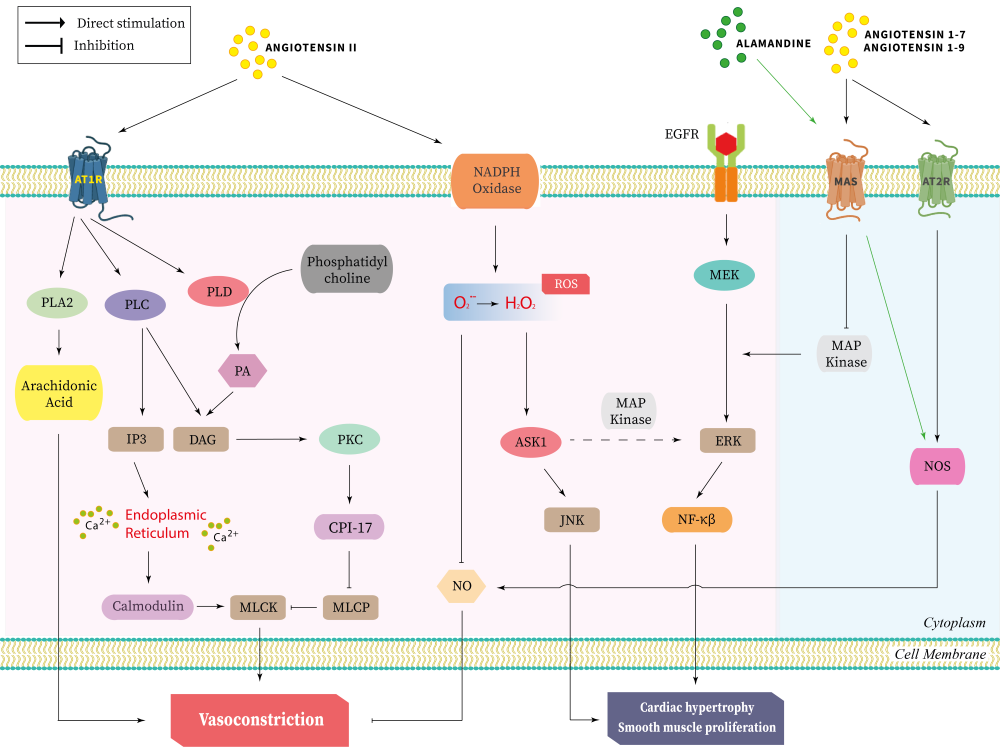
The renin-angiotensin system has emerged as a key modulator of cardiomyopathies, with both local and central effects on the cardiac tissue. With the increase in incidences of cardiac disorders worldwide, the roles of the renin-angiotensin system on the cardiomyopathies have been revealed by scientists. Recent reports suggest that this system might regulate the synthesis of melatonin. This has drawn the interest of scientists globally since melatonin has remarkable protective effects on cardiomyopathies caused by atherosclerosis, cardiac ischemia, and myocardial infarction. Thus, understanding the interactions of melatonin with various components of the renin-angiotensin system becomes a necessary step for devising a targeted therapy for the various cardiomyopathies. This review has summarized the major effects of various components of the renin-angiotensin system on the cardiac tissue and the interaction of this system with melatonin. The role of melatonin in mediating cardioprotective effects by inhibition of certain components of this system has also been discussed.
References
2. Bader M, Peters J, Baltatu O, Müller DN, Luft FC, Ganten D (2001) Tissue renin-angiotensin systems: New insights from experimental animal models in hypertension research. J. Mol. Med. 79 (2): 76–102. DOI: 10.1007/s001090100210.
3. Paz Ocaranza M, Riquelme JA, García L, Jalil JE, Chiong M, Santos RAS, Lavandero S (2020) Counter-regulatory renin–angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 17 (2): 116–129. DOI: 10.1038/s41569-019-0244-8.
4. Bernardi S, Michelli A, Zuolo G, Candido R, Fabris B (2016) Update on RAAS Modulation for the Treatment of Diabetic Cardiovascular Disease. Zhuang Z, editor. J. Diabetes Res. 2016 : 8917578. DOI: 10.1155/2016/8917578.
5. Takahashi H, Yoshika M, Komiyama Y, Nishimura M (2011) The central mechanism underlying hypertension: A review of the roles of sodium ions, epithelial sodium channels, the renin-angiotensin-aldosterone system, oxidative stress and endogenous digitalis in the brain. Hypertens Res. 34 (11): 1147–1160. DOI: 10.1038/hr.2011.105.
6. Sun Y (2010) Intracardiac renin-angiotensin system and myocardial repair/remodeling following infarction. J. Mol. Cell. Cardiol. 48 (3): 483–489. DOI: 10.1016/j.yjmcc.2009.08.002.
7. Agrawal V, Gupta JK, Qureshi SS, Vishwakarma VK (2016) Role of cardiac renin angiotensin system in ischemia reperfusion injury and preconditioning of heart. Indian Heart J. 68 (6): 856–861. DOI: 10.1016/j.ihj.2016.06.010.
8. WC DM (2017) Local Renin Angiotensin Aldosterone Systems and Cardiovascular Diseases. Med. Clin. North Am. 101 (1): 117–127. DOI: 10.1016/j.mcna.2016.08.017.
9. Epstein BJ, Leonard PT, Shah NK (2012) The evolving landscape of RAAS inhibition: From ACE inhibitors to ARBs, to DRIs andbeyond. Expert Rev. Cardiovasc. Ther. 10 (6): 713–725. DOI: 10.1586/erc.12.63.
10. Esteras R, Perez-Gomez MV, Rodriguez-Osorio L, Ortiz A, Fernandez-Fernandez B (2015) Combination use of medicines from two classes of renin-angiotensin system blocking agents: risk of hyperkalemia, hypotension, and impaired renal function. Ther. Adv. drug Saf. 6 (4): 166–176. DOI: 10.1177/2042098615589905.
11. Mei M, Zhou Z, Zhang Q, Chen Y, Zhao H, Shen B (2021) Dual Blockade of the Renin-Angiotensin System: A Strategy that Should Be Reconsidered in Cardiorenal Diseases? Nephron 145 (2): 99–106. DOI: 10.1053/j.ajkd.2008.11.021.
12. Weir MR, Rolfe M (2010) Potassium Homeostasis and Renin-Angiotensin-Aldosterone System Inhibitors. Clin. J. Am. Soc. Nephrol. 5 (3): 531–548. DOI: 10.2215/CJN.07821109.
13. Esquifino AI, Pandi-Perumal SR, Cardinali DP (2004) Circadian organization of the immune response: a role for melatonin. Clin. Appl. Immunol. Rev. 4 (6): 423-433. DOI: 10.1016/j.cair.2004.08.002.
14. Sadeghi M, Khosrawi S, Heshmat-Ghahdarijani K, Gheisari Y, Roohafza H, Mansoorian M, Hoseini SG (2020) Effect of melatonin on heart failure: design for a double-blinded randomized clinical trial. ESC Heart Fail. 7 (5): 3142–3150. DOI: 10.1002/ehf2.12829.
15. Paul M, Poyan Mehr A, Kreutz R (2006) Physiology of Local Renin-Angiotensin Systems. Physiol. Rev. 86 (3): 747–803. DOI: 10.1152/physrev.00036.2005.
16. Dostal DE, Baker KM (1999) The cardiac renin-angiotensin system - conceptual, or a regulator of cardiac function? Circ. Res. 85 (7): 643–650. DOI: 10.1161/01.res.85.7.643.
17. Dostal DE, Baker KM (1999) The cardiac renin-angiotensin system. Circ. Res. 85 (7): 643–650. DOI: 10.1161/01.RES.85.7.643.
18. Endo-Mochizuki Y, Mochizuki N, Sawa H, Takada A, Okamoto H, Kawaguchi H, Nagashima K, Kitabatake A (1995) Expression of renin and angiotensin-converting enzyme in human hearts. Heart Vessels 10 (6): 285–293. DOI: 10.1007/BF02911386.
19. Sun Y, Zhang J, Zhang JQ, Weber KT (2001) Renin expression at sites of repair in the infarcted rat heart. J. Mol. Cell. Cardiol. 33 (5): 995–1003. DOI: 10.1006/jmcc.2001.1365.
20. RC P, JF S, MJ V, MJ D (1996) Expression and localization of renin and angiotensinogen in rat heart after myocardial infarction. Am. J. Physiol. 271 (3 Pt 2). DOI: 10.1152/ajpheart.1996.271.3.H1040.
21. Sawa H, Tokuchi F, Mochizuki N, Endo Y, Furuta Y, Shinohara T, Takada A, Kawaguchi H, Yasuda H, Nagashima K (1992) Expression of the angiotensinogen gene and localization of its protein in the human heart. Circulation 86 (1): 138–146. DOI: 10.1161/01.CIR.86.1.138.
22. Mazzolai, L., Nussberger, J., Aubert, J. F., Brunner, D. B., Gabbiani, G., Brunner, H. R., & Pedrazzini, T (1998) Blood pressure-independent cardiac hypertrophy induced by locally activated renin-angiotensin system. Hypertension 31 (6): 1324–1330. DOI: 10.1161/01.hyp.31.6.1324.
23. Patel VB, Zhong J-C, Grant MB, Oudit GY (2016) Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ. Res. 118 (8): 1313–1326. DOI: 10.1161/CIRCRESAHA.116.307708.
24. Wang W, Bodiga S, Das SK, Lo J, Patel V, Oudit GY (2012) Role of ACE2 in diastolic and systolic heart failure. Heart Fail. Rev. 17 (4): 683–691. DOI: 10.1007/s10741-011-9259-x
25. Duncan, A.M., Burrell, L.M., Kladis, A. and Campbell, D.J. (1996) Effects of angiotensin-converting enzyme inhibition on angiotensin and bradykinin peptides in rats with myocardial infarction. J. Cardiovasc. Pharmacol. , 28 (6), 746 –754. DOI: 10.1097/00005344-199612000-00003.
26. Schmermund A, Lerman LO, Ritman EL, Rumberger JA (1999) Cardiac production of angiotensin II and its pharmacologic inhibition: Effects on the coronary circulation. Mayo Clin. Proc. 74 (5): 503–513. DOI: Https://doi.org/10.4065/74.5.503.
27. Endtmann C, Ebrahimian T, Czech T, Arfa O, Laufs U, Fritz M, Wassmann K, Werner N, Petoumenos V, Nickenig G, Wassmann S (2011) Angiotensin II impairs endothelial progenitor cell number and function in vitro and in vivo. Hypertension 58 (3): 394–403. DOI: 10.1161/HYPERTENSIONAHA.110.169193.
28. Welch WJ (2008) Angiotensin II–dependent superoxide effects on hypertension and vascular dysfunction. Hypertension 52 (1): 51–56. DOI: 10.1161/HYPERTENSIONAHA.107.090472.
29. Schnee JM, Hsueh WA (2000) Angiotensin II, adhesion, and cardiac fibrosis. Cardiovasc. Res. 46 (2): 264–268. DOI: 10.1016/S0008-6363(00)00044-4.
30. Ma TK, Kam KK, Yan BP, Lam YY (2010) Renin-angiotensin-aldosterone system blockade for cardiovascular diseases: current status. Br. J. Pharmacol. 160 (6): 1273–1292. DOI: 10.1111/j.1476-5381.2010.00750.x.
31. Santos RA (2014) Angiotensin-(1–7). Hypertension 63 (6): 1138–1147. DOI: 10.1161/HYPERTENSIONAHA.113.01274.
32. McKinney CA, Fattah C, Loughrey CM, Milligan G, Nicklin SA (2014) Angiotensin-(1–7) and angiotensin-(1–9): function in cardiac and vascular remodelling. Clin. Sci. 126 (12): 815–827. DOI: 10.1042/CS20130436.
33. Jiang F, Yang J, Zhang Y, Dong M, Wang S, Zhang Q, Liu FF, Zhang K, Zhang C (2014) Angiotensin-converting enzyme 2 and angiotensin 1-7: novel therapeutic targets. Nat. Rev. Cardiol. 11 (7): 413–426. DOI: 10.1038/nrcardio.2014.59.
34. Santos RAS, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M, Campagnole-Santos MJ (2018) The ACE2/angiotensin-(1-7)/MAS axis of the renin-angiotensin system: Focus on angiotensin-(1-7). Physiol. Rev. 98 (1): 505–553. DOI: 10.1152/physrev.00023.2016.
35. Schleifenbaum J (2019) Alamandine and its receptor MrgD pair up to join the protective arm of the renin-angiotensin system. Front. Med. 6 (107): 1-6. DOI: 10.3389/fmed.2019.00107.(volume and pager!)
36. Park BM, Phuong HTA, Yu L, Kim SH (2018) Alamandine protects the heart against reperfusion injury via the MrgD receptor. Circ. J. 82 (10): 2584–2593. DOI: 10.1253/circj.CJ-17-138.
37. Silva MM, de Souza-Neto FP, Jesus ICG de, Gonçalves GK, Santuchi M de C, Sanches B de L, de Alcântara-Leonídio TC, Melo MB, Vieira MAR, Guatimosim S, Santos RAS, da Silva RF (2021) Alamandine improves cardiac remodeling induced by transverse aortic constriction in mice. Am. J. Physiol. Circ. Physiol. 320 (1): H352–H363. DOI: 10.1152/ajpheart.00328.2020.
38. Agrawal V, Gupta JK, Qureshi SS, Vishwakarma VK (2016) Role of cardiac renin angiotensin system in ischemia reperfusion injury and preconditioning of heart. Indian Heart J. 68 (6): 856-861. DOI: 10.1016/j.ihj.2016.06.010.
39. Schmidt-Ott KM, Kagiyama S, Phillips MI (2000) The multiple actions of angiotensin II in atherosclerosis. Regul. Pept. 93 (1): 65–77. DOI: 10.1016/s0167-0115(00)00178-6.
40. Mattiazzi A (1997) Positive inotropic effect of angiotensin II increases in intracellular Ca2+ or changes in myofilament Ca2+ responsiveness? J. Pharmacol. Toxicol. Methods 37 (4): 205–214. DOI: 10.1016/s1056-8719(97)00020-8.
41. Berk BC, Corson MA (1997) Angiotensin II signal transduction in vascular smooth muscle. Circ. Res. 80 (5): 607–616. DOI: 10.1161/01.res.80.5.607.
42. Griendling KK, Ushio-Fukai M, Lassègue B, Alexander RW (1997) Angiotensin II signaling in vascular smooth muscle. Hypertension 29 (1): 366–370. DOI: 10.1161/01.hyp.29.1.366.
43. Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, Scalia R, Eguchi S (2018) Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology. Physiol. Rev. 98 (3): 1627–1738. DOI: 10.1152/physrev.00038.2017.
44. Falkenburger BH, Dickson EJ, Hille B (2013) Quantitative properties and receptor reserve of the DAG and PKC branch of Gq-coupled receptor signaling. J. Gen. Physiol. 141 (5): 537–555. DOI: 10.1085/jgp.201210887.
45. Liao D-F, Monia B, Dean N, Berk BC (1997) Protein kinase C-ζ mediates angiotensin II activation of ERK1/2 in vascular smooth muscle cells. J. Biol. Chem. 272 (10): 6146–6150. DOI: 10.1074/jbc.272.10.6146.
46. Feschenko MS, Sweadner KJ (1997) Phosphorylation of Na,K-ATPase by protein kinase C at Ser18 occurs in intact cells but does not result in direct inhibition of atp hydrolysis. J. Biol. Chem. 272 (28): 17726–17733. DOI: 10.1074/jbc.272.28.17726
47. Shmueli E, Alberti KGMM, RECORD CO (1993) Diacylglycerol/protein kinase C signalling: a mechanism for insulin resistance? J. Intern. Med. 234 (4): 397–400. DOI: 10.1111/j.1365-2796.1993.tb00761.x.
48. Park K, Kim J, Nagashima Y, Kako K, Daitoku H, Matsui M, Park GG, Fukamizu A (2014) Detection of choline and phosphatidic acid (PA) catalyzed by phospholipase D (PLD) using MALDI-QIT-TOF/MS with 9-aminoacridine matrix. Biosci. Biotechnol. Biochem. 78 (6): 981–988. DOI: 10.1080/09168451.2014.910102.
49. Arimoto T, Takeishi Y, Takahashi H, Shishido T, Niizeki T, Koyama Y, Shiga R, Nozaki N, Nakajima O, Nishimaru K, Abe J, Endoh M, Walsh RA, Goto K, Kubota I (2006) Cardiac-specific overexpression of diacylglycerol kinase ζ prevents Gq protein-coupled receptor agonist-induced cardiac hypertrophy in transgenic mice. Circulation 113 (1): 60–66. DOI: 10.1161/CIRCULATIONAHA.105.560771.
50. Khalil RA (2013) Protein kinase C inhibitors as modulators of vascular function and their application in vascular disease. Pharmaceuticals (Basel). 6 (3): 407–439. DOI: 10.3390/ph6030407.
51. Khan NS, Song CY, Jennings BL, Estes AM, Fang XR, Bonventre J V, Malik KU (2015) Cytosolic phospholipase A2α is critical for angiotensin II-induced hypertension and associated cardiovascular pathophysiology. Hypertens 65 (4): 784–792. DOI: 10.1161/HYPERTENSIONAHA.114.04803.
52. Kanazawa H, Kurihara N, Hirata K, Kudoh S, Fujii T, Tanaka S, Takeda T (1995) Angiotensin II stimulates peptide leukotriene production by guinea pig airway via the AT1 receptor pathway. Prostaglandins, Leukot. Essent. Fat Acids 52 (4): 241–244. DOI: 10.1016/0952-3278(95)90043-8.
53. Queiroz T, Monteiro M, Braga V (2013) Angiotensin-II-derived reactive oxygen species on baroreflex sensitivity during hypertension: new perspectives. Front. Physiol. 4: 105. DOI: 10.3389/fphys.2013.00105.
54. Miller FJ, Gutterman DD, Rios CD, Heistad DD, Davidson BL (1998) Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ. Res. 82 (12): 1298–1305. DOI: 10.1161/01.res.82.12.1298.
55. Peluffo G, Calcerrada P, Piacenza L, Pizzano N, Radi R (2009) Superoxide-mediated inactivation of nitric oxide and peroxynitrite formation by tobacco smoke in vascular endothelium: studies in cultured cells and smokers. Am. J. Physiol. Heart Circ. Physiol. 296 (6): H1781–H1792. DOI: 10.1152/ajpheart.00930.2008.
56. Reustle A, Torzewski M (2018) Role of p38 MAPK in atherosclerosis and aortic valve sclerosis. Int. J. Mol. Sci. 19 (12): 3761. DOI: 10.3390/ijms19123761.
57. Meijer CA, Le Haen PAA, van Dijk RA, Hira M, Hamming JF, van Bockel JH, Lindeman JH (2012) Activator protein-1 (AP-1) signalling in human atherosclerosis: results of a systematic evaluation and intervention study. Clin. Sci. (Lond). 122 (9): 421–428. DOI: 10.1042/CS20110234.
58. de Winther MPJ, Kanters E, Kraal G, Hofker MH (2005) Nuclear factor κB signaling in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 25 (5): 904–914. DOI: 10.1161/01.ATV.0000160340.72641.87.
59. Mimura J, Itoh K (2015) Role of Nrf2 in the pathogenesis of atherosclerosis. Free Radic. Biol. Med. 88 : 221–232. DOI: 10.1161/01.ATV.0000160340.72641.87.
60. Sato A, Okada M, Shibuya K, Watanabe E, Seino S, Narita Y, Shibui S, Kayama T, Kitanaka C (2014) Pivotal role for ROS activation of p38 MAPK in the control of differentiation and tumor-initiating capacity of glioma-initiating cells. Stem Cell Res. 12 (1): 119–131. DOI: 10.1016/j.scr.2013.09.012.
61. Aharoni-Simon M, Reifen R, Tirosh O (2006) ROS-production–mediated activation of AP-1 but not NFκB inhibits glutamate-induced HT4 neuronal cell death. Antioxid. Redox Signal 8 (7–8): 1339–1349. DOI: 10.1089/ars.2006.8.1339.
62. Frohlich DA, McCabe MT, Arnold RS, Day ML (2008) Role of Nrf2 in the pathogenesis of atherosclerosis. Oncogene 27 (31): 4353–4362. DOI: 10.1016/j.freeradbiomed.2015.06.019.
63. Lockyer JM, Colladay JS, Alperin-Lea WL, Hammond T, Buda AJ (1998) Inhibition of nuclear factor-κB–mediated adhesion molecule expression in human endothelial cells. Circ. Res. 82 (3): 314–320. DOI: 10.1161/01.res.82.3.314.
64. Kudoh S, Komuro I, Mizuno T, Yamazaki T, Zou Y, Shiojima I, Takekoshi N, Yazaki Y (1997) Angiotensin II stimulates c-Jun NH2-terminal kinase in cultured cardiac myocytes of neonatal rats. Circ. Res. 80 (1): 139–146. DOI: 10.1161/01.res.80.1.139.
65. Rose, B. A., Force, T., & Wang, Y (2010) Mitogen-activated protein kinase signaling in the heart: Angels versus demons in a heart-breaking tale. Physiol. Rev. 90 (4): 1507–1546. DOI: 10.1152/physrev.00054.2009.
66. Roberts RE (2012) The extracellular signal-regulated kinase (ERK) pathway: a potential therapeutic target in hypertension. J. Exp. Pharmacol. 4 : 77–83.
67. Manzoor Z, Koh YS (2012) Mitogen-activated protein kinases in inflammation. J. Bacteriol. Virol. 42 (3): 189–195. DOI: 10.4167/jbv.2012.42.3.189.
68. Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y (1997) Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275 (5296): 90–94. DOI: 10.1126/science.275.5296.90.
69. Izumiya Y, Kim S, Izumi Y, Yoshida K, Yoshiyama M, Matsuzawa A, Ichijo H, Iwao H (2003) Apoptosis signal-regulating kinase 1 plays a pivotal role in angiotensin II–Induced cardiac hypertrophy and remodeling. Circ. Res. 93 (9): 874–883. DOI: 10.1161/01.RES.0000100665.67510.F5.
70. Peng K, Tian X, Qian Y, Skibba M, Zou C, Liu Z, Wang J, Xu Z, Li X, Liang G (2016) Novel EGFR inhibitors attenuate cardiac hypertrophy induced by angiotensin II. J. Cell. Mol. Med. 20 (3): 482–494. DOI: 10.1111/jcmm.12763.
71. Cha SA, Park BM, Kim SH (2018) Angiotensin-(1-9) ameliorates pulmonary arterial hypertension via angiotensin type II receptor. Korean J. Physiol. Pharmacol. 22 (4): 447–456. DOI: 10.4196/kjpp.2018.22.4.447.
72. Sampaio WO, dos Santos RAS, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM (2007) Angiotensin-(1-7) through receptor mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension 49 (1): 185–192. DOI: 10.1161/01.HYP.0000251865.35728.2f.
73. Gomes ERM, Santos RAS, Guatimosim S (2012) Angiotensin-(1-7)-mediated signaling in cardiomyocytes. Int. J. Hypertens 2012 : 493129. DOI: 10.1155/2012/493129.
74. Zhang F, Ren X, Zhao M, Zhou B, Han Y (2016) Angiotensin-(1–7) abrogates angiotensin II-induced proliferation, migration and inflammation in VSMCs through inactivation of ROS-mediated PI3K/Akt and MAPK/ERK signaling pathways. Sci. Rep. 6 (1): 34621. DOI: 0.1038/srep34621.
75. Sampaio WO, de Castro CH, Santos RAS, Schiffrin EL, Touyz RM (2007) Angiotensin-(1-7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension 50 (6): 1093–1098. DOI: 10.1161/HYPERTENSIONAHA.106.084848.
76. Tallant EA, Ferrario CM, Gallagher PE (2005) Angiotensin-(1–7) inhibits growth of cardiac myocytes through activation of the mas receptor. Am. J. Physiol. Circ. Physiol. 289 (4): H1560–H1566. DOI: 10.1152/ajpheart.00941.2004.
77. Grobe JL, Mecca AP, Lingis M, Shenoy V, Bolton TA, Machado JM, Speth RC, Raizada MK, Katovich MJ (2007) Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1–7). Am. J. Physiol. Circ. Physiol. 292 (2): H736–H742. DOI: 10.1152/ajpheart.00937.2006.
78. Flores-Munoz M, Work LM, Douglas K, Denby L, Dominiczak AF, Graham D, Nicklin SA (2012) Angiotensin-(1-9) attenuates cardiac fibrosis in the stroke-prone spontaneously hypertensive rat via the angiotensin type 2 receptor. Hypertension 59 (2): 300–307. DOI: 10.1161/HYPERTENSIONAHA.111.177485.
79. Zhao J, Gao J-L, Zhu J-X, Zhu H-B, Peng X, Jiang M, Fu Y, Xu J, Mao X-H, Hu N, Ma M-H, Dong D-L (2019) The different response of cardiomyocytes and cardiac fibroblasts to mitochondria inhibition and the underlying role of STAT3. Basic Res. Cardiol. 114 (2): 12. DOI: 10.1007/s00395-019-0721-6.
80. Zhou H, Li N, Yuan Y, Jin Y-G, Guo H, Deng W, Tang Q-Z (2018) Activating transcription factor 3 in cardiovascular diseases: A potential therapeutic target. Basic Res. Cardiol. 113 (5): 37. DOI: 10.1007/s00395-018-0698-6.
81. Nofer JR, Brodde MF, Kehrel BE (2010) High-density lipoproteins, platelets and the pathogenesis of atherosclerosis: Frontiers in research review: Physiological and pathological functions of high-density lipoprotein. Clin. Exp. Pharmacol. Physiol. 37 (7): 726–735. DOI: 10.1111/j.1440-1681.2010.05377.x.
82. Fu Z, Jiao Y, Wang J, Zhang Y, Shen M, Reiter RJ, Xi Q, Chen Y (2020) Cardioprotective role of melatonin in acute myocardial infarction. Front. Physiol. 11 (366). DOI: 10.3389/fphys.2020.00366.
83. Thieltges KM, Avramovic D, Piscitelli CL, Markovic-Mueller S, Binz HK, Ballmer-Hofer K (2018) Characterization of a drug-targetable allosteric site regulating vascular endothelial growth factor signaling. Angiogenesis 21 (3): 533–543. DOI: 10.1007/s10456-018-9606-9.
84. Chen W, Spitzl A, Mathes D, Nikolaev VO, Werner F, Weirather J, Špiranec K, Röck K, Fischer JW, Kämmerer U, Stegner D, Baba HA, Hofmann U, Frantz S, Kuhn M (2016) Endothelial Actions of ANP enhance myocardial inflammatory infiltration in the early phase after acute infarction. Circ. Res. 119 (2): 237–248. DOI: 10.1161/CIRCRESAHA.115.307196
85. Datta M, Majumder R, Chattopadhyay A, Bandyopadhyay D (2021) Protective effect of melatonin in atherosclerotic cardiovascular disease: A comprehensive review. Melatonin Res. 4 (3): 408–430. DOI: 10.32794/mr112500102.
86. Tripathi KD 2004. [Internet] (2004) Essentials of medical pharmacology. Jaypee Brothers, Medical Publishers; 2004. 184–185 p. DOI: 10.5005/jp/books/12256.
87. Lerner AB, Case JD, Mori W, Wright MR (1959) Melatonin in peripheral nerve. Nature 183 (4678): 1821. DOI: 10.1038/1831821a0.
88. Lochner A, Marais E, Huisamen B (2018) Melatonin and cardioprotection against ischaemia/reperfusion injury: What’s new? A review. J. Pineal Res. 65 (1). DOI: 10.1111/jpi.12490.
89. Yang Y, Sun Y, Yi W, Li Y, Fan C, Xin Z, Jiang S, Di S, Qu Y, Reiter RJ, Yi D (2009) A review of melatonin as a suitable antioxidant against myocardial ischemia-reperfusion injury and clinical heart diseases. J. Pineal Res. 57 (4): 357–366. DOI: 10.1111/jpi.12175.
90. Ding S, Lin N, Sheng X, Zhao Y, Su Y, Xu L, Tong R, Yan Y, Fu Y, He J, Gao Y, Yuan A, Ye L, Reiter RJ, Pu J (2019) Melatonin stabilizes rupture-prone vulnerable plaques via regulating macrophage polarization in a nuclear circadian receptor RORα-dependent manner. J. Pineal Res. 67 (2). DOI: 10.1111/jpi.12581.
91. Soto-Heras S, Catalá M-G, Roura M, Menéndez-Blanco I, Piras A-R, Izquierdo D, Paramio M-T (2019) Effects of melatonin on oocyte developmental competence and the role of melatonin receptor 1 in juvenile goats. Reprod. Domest. Anim. 54 (2): 381–390. DOI: 10.1111/rda.13378.
92. Baltatu O, Afeche SC, Santos SHJ dos, Campos LA, Barbosa R, Michelini LC, Bader M, Cipolla-Neto J (2002) Locally synthesized angiotensin modulates pineal melatonin generation. J. Neurochem. 80 (2): 328–334. DOI: 10.1046/j.0022-3042.2001.00701.x.
93. Wang G, Chen X, Zhang C, Li M, Sun C, Zhan N, Huang X, Li T, Deng W (2021) Biosynthetic pathway and the potential role of melatonin at different abiotic stressors and developmental stages in tolypocladium guangdongense. Front. Microbiol. 12: 746141 DOI: 10.3389/fmicb.2021.746141.
94. Galano A, Tan DX, Reiter RJ (2011) Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 51 (1): 1–16. DOI: 10.1111/j.1600-079X.2011.00916.x.
95. Sun H, Gusdon AM, Qu S (2016) Effects of melatonin on cardiovascular diseases: progress in the past year. Curr. Opin. Lipidol. 27 (4): 408–413. DOI: 10.1097/MOL.0000000000000314.
96. Reiter RJ, Mayo JC, Tan D-X, Sainz RM, Alatorre-Jimenez M, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 61 (3): 253–278. DOI: 10.1111/jpi.12360.
97. Luo G-P, Jian Z, Ma R-Y, Cao Z-Z, Zhu Y, Zhu Y, Tang F-Q, Xiao Y-B (2018) Melatonin alleviates hypoxia-induced cardiac apoptosis through PI3K/Akt pathway. Int. J. Clin. Exp. Pathol. 11 (12): 5840–5849. PMID: 31949670.
98. Tan D-X, Manchester LC, Esteban-Zubero E, Zhou Z, Reiter RJ (2015) Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules 20 (10): 18886–18906. DOI: 10.3390/molecules201018886.
99. Che H, Wang Y, Li H, Li Y, Sahil A, Lv J, Liu Y, Yang Z, Dong R, Xue H, Wang L (2020) Melatonin alleviates cardiac fibrosis via inhibiting lncRNA MALAT1/miR-141-mediated NLRP3 inflammasome and TGF-β1/Smads signaling in diabetic cardiomyopathy. FASEB J. 34 (4): 5282–5298. DOI: 10.1096/fj.201902692R.
100. Qi X, Wang J (2020) Melatonin improves mitochondrial biogenesis through the AMPK/PGC1α pathway to attenuate ischemia/reperfusion-induced myocardial damage. Aging (Albany. NY). 12 (8): 7299–7312. DOI: 10.18632/aging.103078.
101. Zhou J, Zhang S, Zhao X, Wei T (2008) Melatonin impairs NADPH oxidase assembly and decreases superoxide anion production in microglia exposed to amyloid-β1-42. J. Pineal Res. 45 (2): 157–165. DOI: 10.1111/j.1600-079X.2008.00570.x.
102. Doolen S, Krause DN, Dubocovich ML, Duckles SP (1998) Melatonin mediates two distinct responses in vascular smooth muscle. Eur. J. Pharmacol. 345 (1): 67–69. DOI: 10.1016/s0014-2999(98)00064-8.
103. Veneroso C, Tuñón MJ, González-Gallego J, Collado PS (2009) Melatonin reduces cardiac inflammatory injury induced by acute exercise. J. Pineal Res. 47 (2): 184–191. DOI: 10.1111/j.1600-079X.2009.00699.x.
104. Reiter RJ, Calvo JR, Karbownik M, Qi W, Tan DX (2000) Melatonin and its relation to the immune system and inflammation. Ann. N. Y. Acad. Sci. 917: 376–386. DOI: 10.1111/j.1749-6632.2000.tb05402.x.
105. Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SE, Mossialos EA, Maggioni AP, Kazakiewicz D, May HT, De Smedt D (2020) European Society of Cardiology: cardiovascular disease statistics 2019. Eur. Heart J. 41(1):12-85. DOI: 10.1093/eurheartj/ehz859

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


