Ameliorating effects of melatonin on high-fat diet induced non-alcoholic fatty liver diseases and their associated pathologies: A comprehensive review
Protective role of melatonin on HFD induced NAFLD
Abstract
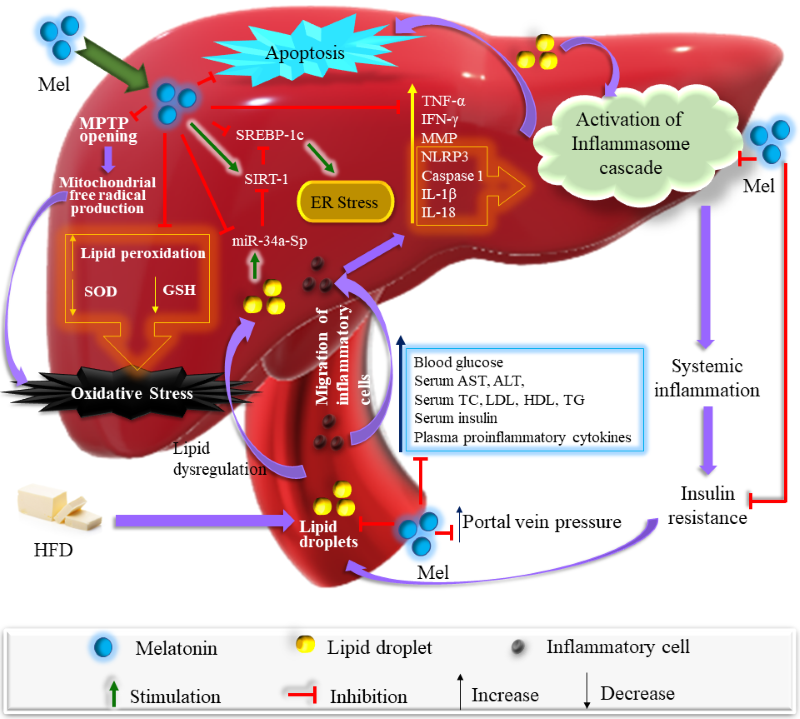
Non-alcoholic fatty liver disease (NAFLD) is caused by hepatic fat accumulation with a high prevalence globally, especially in Western countries in which individuals have excessive fat consumption. Prolonged intake of high dietary fat causes various diseases due to the imbalance of energy metabolism, which leads to obesity and other pathological conditions. Currently, the exact pathogenesis of NAFLD is still obscure. In this review, the potential etiologies for NAFLD will be discussed, including adipose tissue dysfunction, intrahepatic de novo lipogenesis, hepatic fat accumulation, insulin resistance, hepatic inflammation, inflammasome activation, mitochondrial dysfunction, oxidative stress, and endoplasmic reticulum stress. Melatonin is a potent antioxidant and anti-inflammatory molecule. It is also a regulator of lipid and glucose metabolism which is indicated by melatonin’s effects on weight loss, reduction of liver weight, blood levels of lipids, glucose and insulin, activities of hepatic enzymes, steatohepatitis, and fibrosis. Melatonin considerably reduces mitochondrial dysfunction and proinflammatory cytokines. Moreover, it downregulates NLRP3 and its associated downstream effectors of caspase-1, IL-1β, and IL-18 proteins. This review will update the molecular mechanisms behind high-fat diet induced hepatic dysfunction and the protective role of melatonin in NAFLD.
References
2. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M (2016) Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64 (1): 73-84. doi: 10.1002/hep.28431.
3. Højland Ipsen D, Tveden-Nyborg P, Lykkesfeldt J (2016) Normal weight dyslipidemia: Is it all about the liver? Obesity (Silver Spring). 24 (3): 556-567. doi: 10.1002/oby.21443.
4. Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P (2018) Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol. Life Sci. 75 (18): 3313-3327. doi: 10.1007/s00018-018-2860-6.
5. Than NN, Newsome PN (2015) A concise review of non-alcoholic fatty liver disease. Atherosclerosis 239 (1): 192-202. doi: 10.1016/j.atherosclerosis.2015.01.001.
6. Carmiel-Haggai M, Cederbaum AI, Nieto N (2005) A high-fat diet leads to the progression of non-alcoholic fatty liver disease in obese rats. FASEB J. 19 (1): 136-8. doi: 10.1096/fj.04-2291fje.
7. Angulo P (2002) Nonalcoholic fatty liver disease. N. Engl. J. Med. 346 (16): 1221-1231. doi: 10.1056/NEJMra011775. doi: 10.1001/jama.2015.5370.
8. Rinella ME (2015) Nonalcoholic fatty liver disease: a systematic review. JAMA 313 (22): 2263-2273. doi: 10.1039/c9fo01611b.
9. Hu Y, Yin F, Liu Z, Xie H, Xu Y, Zhou D, Zhu B (2020) Acerola polysaccharides ameliorate high-fat diet-induced non-alcoholic fatty liver disease through reduction of lipogenesis and improvement of mitochondrial functions in mice. Food Funct. 11 (1): 1037-1048. doi: 10.1039/c9fo01611b.
10. Sun H, Huang FF, Qu S (2015) Melatonin: a potential intervention for hepatic steatosis. Lipids Health Dis. 14: 75. doi:10.1186/s12944-015-0081-7.
11. Florido J, Rodriguez-Santana C, Martinez-Ruiz L, López-Rodríguez A, Acuña-Castroviejo D, Rusanova I, Escames G (2022) Understanding the mechanism of action of melatonin, which induces ros production in cancer cells. Antioxidants (Basel). 11 (8): 1621. doi: 10.3390/antiox11081621.
12. Pan M, Song YL, Xu JM, Gan HZ (2006) Melatonin ameliorates nonalcoholic fatty liver induced by high-fat diet in rats. J. Pineal Res. 41 (1): 79-84. doi: 10.1111/j.1600-079X.2006.00346.x.
13. Peschke E. Melatonin, endocrine pancreas and diabetes (2008) J. Pineal Res. 44 (1): 26-40. doi: 10.1111/j.1600-079X.2007.00519.x.
14. Kanter M, Uysal H, Karaca T, Sagmanligil HO (2006) Depression of glucose levels and partial restoration of pancreatic beta-cell damage by melatonin in streptozotocin-induced diabetic rats. Arch. Toxicol. 80 (6): 362-369. doi: 10.1007/s00204-005-0055-z.
15. Nishida S, Sato R, Murai I, Nakagawa S (2003) Effect of pinealectomy on plasma levels of insulin and leptin and on hepatic lipids in type 2 diabetic rats. J. Pineal Res. 35 (4): 251-256. doi: 10.1034/j.1600-079x.2003.00083.x.
16. Nishida S, Segawa T, Murai I, Nakagawa S (2002) Long-term melatonin administration reduces hyperinsulinemia and improves the altered fatty-acid compositions in type 2 diabetic rats via the restoration of Delta-5 desaturase activity. J. Pineal Res. 32 (1): 26-33. doi: 10.1034/j.1600-079x.2002.10797.x.
17. Shieh JM, Wu HT, Cheng KC, Cheng JT (2009) Melatonin ameliorates high fat diet-induced diabetes and stimulates glycogen synthesis via a PKC zeta-Akt-GSK3 beta pathway in hepatic cells. J. Pineal Res. 47 (4): 339-344. doi: 10.1111/j.1600-079X.2009.00720.x.
18. Mells JE, Fu PP, Kumar P, Smith T, Karpen SJ, Anania FA (2015) Saturated fat and cholesterol are critical to inducing murine metabolic syndrome with robust nonalcoholic steatohepatitis. J. Nutr. Biochem. 26 (3): 285-292. doi: 10.1016/j.jnutbio.2014.11.002.
19. Alkhouri N, Dixon LJ, Feldstein AE (2009) Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev. Gastroenterol. Hepatol. 3 (4): 445-451. doi: 10.1586/egh.09.32.
20. Lian CY, Zhai ZZ, Li ZF, Wang L (2020) High fat diet-triggered non-alcoholic fatty liver disease: A review of proposed mechanisms. Chem. Biol. Interact. 330: 109199. doi: 10.1016/j.cbi.2020.109199.
21. Wong MCS, Huang JLW, George J, Huang J, Leung C, Eslam M, Chan HLY, Ng SC (2019) The changing epidemiology of liver diseases in the Asia-Pacific region. Nat. Rev. Gastroenterol. Hepatol. 16 (1): 57-73. doi: 10.1038/s41575-018-0055-0.
22. Fan JG, Kim SU, Wong VW (2017) New trends on obesity and NAFLD in Asia. J. Hepatol. 67 (4): 862-873. doi: 10.1016/j.jhep.2017.06.003.
23. Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, Fujii H, Wu Y, Kam LY, Ji F, Li X, Chien N, Wei M, Ogawa E, Zhao C, Wu X, Stave CD, Henry L, Barnett S, Takahashi H, Furusyo N, Eguchi Y, Hsu YC, Lee TY, Ren W, Qin C, Jun DW, Toyoda H, Wong VW, Cheung R, Zhu Q, Nguyen MH (2019) Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 4 (5): 389-398. doi: 10.1016/S2468-1253(19)30039-1.
24. Mitra S, De A, Chowdhury A (2020) Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl. Gastroenterol. Hepatol. 5:16. doi: 10.21037/tgh.2019.09.08.
25. Duseja A (2010) Nonalcoholic fatty liver disease in India - a lot done, yet more required! Ind, J. Gastroenterol. 29 (6): 217-25. doi: 10.1007/s12664-010-0069-1.
26. Duvnjak L, Duvnjak M (2009) The metabolic syndrome - an ongoing story. J. Physiol. Pharmacol. 7: 19-24. PMID: 20388942.
27. Gonciarz M, Gonciarz Z, Bielanski W, Mularczyk A, Konturek PC, Brzozowski T, Konturek SJ (2010) The pilot study of 3-month course of melatonin treatment of patients with nonalcoholic steatohepatitis: effect on plasma levels of liver enzymes, lipids and melatonin. J. Physiol. Pharmacol. 61 (6): 705-710. PMID: 21224501.
28. Kenneally S, Sier JH, Moore JB (2017) Efficacy of dietary and physical activity intervention in non-alcoholic fatty liver disease: a systematic review. BMJ Open Gastroenterol. 4 (1): e000139. doi: 10.1136/bmjgast-2017-000139.
29. Softic S, Cohen DE, Kahn CR (2016) Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig. Dis. Sci. 61 (5): 1282-1293. doi: 10.1007/s10620-016-4054-0.
30. Jensen VS, Hvid H, Damgaard J, Nygaard H, Ingvorsen C, Wulff EM, Lykkesfeldt J, Fledelius C (2018) Dietary fat stimulates development of NAFLD more potently than dietary fructose in Sprague-Dawley rats. Diabetol. Metab. Syndr. 10: 4. doi: 10.1186/s13098-018-0307-8.
31. Fielding B (2011) Tracing the fate of dietary fatty acids: metabolic studies of postprandial lipaemia in human subjects. Proc. Nutr. Soc. 70 (3): 342-350. doi: 10.1017/S002966511100084X.
32. Lindeboom L, Nabuurs CI, Hesselink MK, Wildberger JE, Schrauwen P, Schrauwen-Hinderling VB (2015) Proton magnetic resonance spectroscopy reveals increased hepatic lipid content after a single high-fat meal with no additional modulation by added protein. Am. J. Clin. Nutr. 101 (1): 65-71. doi: 10.1152/ajpgi.00413.2005.
33. Bradbury MW (2006) Lipid metabolism and liver inflammation. I. Hepatic fatty acid uptake: possible role in steatosis. Am. J. Physiol. Gastrointest Liver Physiol. 290 (2): G194-198. doi: 10.1152/ajpgi.00413.2005.
34. Xenoulis PG, Steiner JM (2010) Lipid metabolism and hyperlipidemia in dogs. Vet. J. 183 (1): 12-21. doi: 10.1016/j.tvjl.2008.10.011.
35. Hirsova P, Ibrabim SH, Gores GJ, Malhi H (2016) Lipotoxic lethal and sublethal stress signaling in hepatocytes: relevance to NASH pathogenesis. J. Lipid Res. 57 (10): 1758-1770. Erratum in: J Lipid Res. 2017 Jan;58(1):299. doi: 10.1194/jlr.R066357.
36. Barreyro FJ, Kobayashi S, Bronk SF, Werneburg NW, Malhi H, Gores GJ (2007) Transcriptional regulation of Bim by FoxO3A mediates hepatocyte lipoapoptosis. J. Biol. Chem. 282 (37): 27141-27154. doi: 10.1074/jbc.M704391200.
37. Musso G, Gambino R, De Michieli F, Cassader M, Rizzetto M, Durazzo M, Fagà E, Silli B, Pagano G (2003) Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 37 (4): 909-916. doi: 10.1053/jhep.2003.50132.
38. Toshimitsu K, Matsuura B, Ohkubo I, Niiya T, Furukawa S, Hiasa Y, Kawamura M, Ebihara K, Onji M (2007) Dietary habits and nutrient intake in non-alcoholic steatohepatitis. Nutrition 23 (1): 46-52. doi: 10.1016/j.nut.2006.09.004.
39. Rosqvist F, Iggman D, Kullberg J, Cedernaes J, Johansson HE, Larsson A, Johansson L, Ahlström H, Arner P, Dahlman I, Risérus U (2014) Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes 63 (7): 2356-2368. doi: 10.2337/db13-1622.
40. Armstrong MJ, Hazlehurst JM, Hull D, Guo K, Borrows S, Yu J, Gough SC, Newsome PN, Tomlinson JW (2014) Abdominal subcutaneous adipose tissue insulin resistance and lipolysis in patients with non-alcoholic steatohepatitis. Diabetes. Obes. Metab. 16 (7): 651-660. doi: 10.1111/dom.12272.
41. Musso G, Cassader M, De Michieli F, Rosina F, Orlandi F, Gambino R (2012) Nonalcoholic steatohepatitis versus steatosis: adipose tissue insulin resistance and dysfunctional response to fat ingestion predict liver injury and altered glucose and lipoprotein metabolism. Hepatology 56 (3): 933-942. doi: 10.1002/hep.25739.
42. Lomonaco R, Ortiz-Lopez C, Orsak B, Webb A, Hardies J, Darland C, Finch J, Gastaldelli A, Harrison S, Tio F, Cusi K (2012) Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology 55 (5): 1389-1397. doi: 10.1016/j.jhep.2013.02.007.
43. Safar Zadeh E, Lungu AO, Cochran EK, Brown RJ, Ghany MG, Heller T, Kleiner DE, Gorden P (2013) The liver diseases of lipodystrophy: the long-term effect of leptin treatment. J. Hepatol. 59 (1): 131-137. doi: 10.1038/ajg.2016.178.
44. Chang Y, Jung HS, Cho J, Zhang Y, Yun KE, Lazo M, Pastor-Barriuso R, Ahn J, Kim CW, Rampal S, Cainzos-Achirica M, Zhao D, Chung EC, Shin H, Guallar E, Ryu S (2016) Metabolically healthy obesity and the development of nonalcoholic fatty liver disease. Am. J. Gastroenterol. 111 (8): 1133-1140. doi: 10.1038/ajg.2016.178.
45. Buechler C, Wanninger J, Neumeier M (2011) Adiponectin, a key adipokine in obesity related liver diseases. World J. Gastroenterol. 17 (23): 2801-2811. doi: 10.3748/wjg.v17.i23.2801.
46. Yoon MJ, Lee GY, Chung JJ, Ahn YH, Hong SH, Kim JB (2006) Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes 55 (9): 2562-2570. doi: 10.2337/db05-1322.
47. Liu Q, Yuan B, Lo KA, Patterson HC, Sun Y, Lodish HF (2012) Adiponectin regulates expression of hepatic genes critical for glucose and lipid metabolism. Proc. Natl. Acad. Sci. USA. 109 (36): 14568-14573. doi: 10.1073/pnas.1211611109.
48. Matsumoto H, Tamura S, Kamada Y, Kiso S, Fukushima J, Wada A, Maeda N, Kihara S, Funahashi T, Matsuzawa Y, Shimomura I, Hayashi N (2006) Adiponectin deficiency exacerbates lipopolysaccharide/D-galactosamine-induced liver injury in mice. World J. Gastroenterol. 12 (21): 3352-3358. doi: 10.3748/wjg.v12.i21.3352.
49. Kumar P, Raeman R, Chopyk DM, Smith T, Verma K, Liu Y, Anania FA (2018) Adiponectin inhibits hepatic stellate cell activation by targeting the PTEN/AKT pathway. Biochim. Biophys. Acta Mol. Basis Dis. 1864 (10): 3537-3545. doi: 10.1016/j.bbadis.2018.08.012.
50. Jamali R, Razavizade M, Arj A, Aarabi MH (2016) Serum adipokines might predict liver histology findings in non-alcoholic fatty liver disease. World J. Gastroenterol. 22 (21): 5096-5103. doi: 10.3748/wjg.v22.i21.5096.
51. Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, Webb A, Portillo-Sanchez P (2016) Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: A randomized trial. Ann. Intern. Med. 165 (5): 305-315. doi: 10.7326/M15-1774.
52. Marjot T, Moolla A, Cobbold JF, Hodson L, Tomlinson JW (2020) Nonalcoholic fatty liver disease in adults: Current concepts in etiology, outcomes, and management. Endocr. Rev. 41 (1): bnz009. doi: 10.1210/endrev/bnz009.
53. Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ (2003) The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J. Clin. Invest. 112 (1): 91-100. doi: 10.1172/JCI17797.
54. Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ (2014) Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 146 (3): 726-735. doi: 10.1053/j.gastro.2013.11.049.
55. Mitsuyoshi H, Yasui K, Harano Y, Endo M, Tsuji K, Minami M, Itoh Y, Okanoue T, Yoshikawa T (2009) Analysis of hepatic genes involved in the metabolism of fatty acids and iron in nonalcoholic fatty liver disease. Hepatol. Res. 39 (4): 366-373. doi: 10.1111/j.1872-034X.2008.00464.x.
56. Ferré P, Foufelle F (2010) Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes. Metab. 2: 83-92. doi: 10.1111/j.1463-1326.2010.01275.x
57. Brown MS, Goldstein JL (2008) Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 7 (2): 95-96. doi: 10.1016/j.cmet.2007.12.009.
58. Schwarz JM, Linfoot P, Dare D, Aghajanian K (2003) Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am. J. Clin. Nutr. 77 (1): 43-50. doi: 10.1093/ajcn/77.1.43.
59. Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL (2000) Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol. Cell 6 (1): 77-86. PMID: 10949029.
60. Pajvani UB, Qiang L, Kangsamaksin T, Kitajewski J, Ginsberg HN, Accili D (2013) Inhibition of Notch uncouples Akt activation from hepatic lipid accumulation by decreasing mTorc1 stability. Nat. Med. 19 (8): 1054-1060. doi: 10.1038/nm.3259.
61. Wu X, Chen K, Williams KJ (2012) The role of pathway-selective insulin resistance and responsiveness in diabetic dyslipoproteinemia. Curr. Opin. Lipidol. 23 (4): 334-344. doi: 10.1097/MOL.0b013e3283544424.
62. Vatner DF, Majumdar SK, Kumashiro N, Petersen MC, Rahimi Y, Gattu AK, Bears M, Camporez JP, Cline GW, Jurczak MJ, Samuel VT, Shulman GI (2015) Insulin-independent regulation of hepatic triglyceride synthesis by fatty acids. Proc. Natl. Acad. Sci. U S A. 112 (4): 1143-1148. doi: 10.1073/pnas.1423952112.
63. Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ (2005) Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 115 (5): 1343-1351. doi: 10.1172/JCI23621.
64. Sevastianova K, Santos A, Kotronen A, Hakkarainen A, Makkonen J, Silander K, Peltonen M, Romeo S, Lundbom J, Lundbom N, Olkkonen VM, Gylling H, Fielding BA, Rissanen A, Yki-Järvinen H (2012) Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans. Am. J. Clin. Nutr. 96 (4): 727-734. doi: 10.3945/ajcn.112.038695.
65. Flannery C, Dufour S, Rabøl R, Shulman GI, Petersen KF (2012) Skeletal muscle insulin resistance promotes increased hepatic de novo lipogenesis, hyperlipidemia, and hepatic steatosis in the elderly. Diabetes 61 (11): 2711-2717. doi: 10.2337/db12-0206.
66. DeFronzo RA, Tripathy D (2009) Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 32: 157-163. doi: 10.2337/dc09-S302.
67. Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR (2000) Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol. Cell 6 (1): 87-97. PMID: 10949030.
68. Hardy T, Oakley F, Anstee QM, Day CP (2016) Nonalcoholic Fatty Liver Disease: Pathogenesis and Disease Spectrum. Annu. Rev. Pathol. 11: 451-496. doi: 10.1146/annurev-pathol-012615-044224.
69. Kraegen EW, Clark PW, Jenkins AB, Daley EA, Chisholm DJ, Storlien LH (1991) Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes 40 (11): 1397-1403. doi: 10.2337/diab.40.11.1397.
70. VanSaun MN, Lee IK, Washington MK, Matrisian L, Gorden DL (2009) High fat diet induced hepatic steatosis establishes a permissive microenvironment for colorectal metastases and promotes primary dysplasia in a murine model. Am. J. Pathol. 175 (1): 355-364. doi: 10.2353/ajpath.2009.080703.
71. Nakamura A, Terauchi Y (2013) Lessons from mouse models of high-fat diet-induced NAFLD. Int. J. Mol. Sci. 14 (11): 21240-21257. doi: 10.3390/ijms141121240.
72. Du J, Zhang M, Lu J, Zhang X, Xiong Q, Xu Y, Bao Y, Jia W (2016) Osteocalcin improves nonalcoholic fatty liver disease in mice through activation of Nrf2 and inhibition of JNK. Endocrine 53 (3): 701-709. doi: 10.1007/s12020-016-0926-5.
73. Wang H, Zhu YY, Wang L, Teng T, Zhou M, Wang SG, Tian YZ, Du L, Yin XX, Sun Y (2017) Mangiferin ameliorates fatty liver via modulation of autophagy and inflammation in high-fat-diet induced mice. Biomed. Pharmacother. 96: 328-335. doi: 10.1016/j.biopha.2017.10.022.
74. Jeong HS, Kim KH, Lee IS, Park JY, Kim Y, Kim KS, Jang HJ (2017) Ginkgolide A ameliorates non-alcoholic fatty liver diseases on high fat diet mice. Biomed. Pharmacother. 88: 625-634. doi: 10.1016/j.biopha.2017.01.114.
75. Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL (2000) Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol. Cell 6 (1): 77-86. PMID: 10949029.
76. Biddinger SB, Haas JT, Yu BB, Bezy O, Jing E, Zhang W, Unterman TG, Carey MC, Kahn CR (2008) Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat. Med. 14 (7): 778-782. doi: 10.1038/nm1785.
77. Brown MS, Goldstein JL (2008) Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 7 (2): 95-96. doi: 10.1016/j.cmet.2007.12.009.
78. Li S, Brown MS, Goldstein JL (2010) Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc. Natl. Acad. Sci. USA. 107 (8): 3441-3446. doi: 10.1073/pnas.0914798107.
79. Neuschwander-Tetri BA (2010) Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology 52 (2): 774-788. doi: 10.1002/hep.23719.
80. Crespo J, Cayón A, Fernández-Gil P, Hernández-Guerra M, Mayorga M, Domínguez-Díez A, Fernández-Escalante JC, Pons-Romero F (2001) Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology 34 (6): 1158-1163. doi: 10.1053/jhep.2001.29628.
81. Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE (2005) Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 11 (2):183-190. doi: 10.1038/nm1166.
82. Ribeiro PS, Cortez-Pinto H, Solá S, Castro RE, Ramalho RM, Baptista A, Moura MC, Camilo ME, Rodrigues CM (2004) Hepatocyte apoptosis, expression of death receptors, and activation of NF-κβ in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am. J. Gastroenterol. 99 (9):1708-1717. doi: 10.1111/j.1572-0241.2004.40009.x.
83. Arrese M, Cabrera D, Kalergis AM, Feldstein AE (2016) Innate Immunity and Inflammation in NAFLD/NASH. Dig. Dis. Sci. 61 (5):1294-1303. doi: 10.1007/s10620-016-4049-x.
84. Petrasek J, Csak T, Szabo G (2013) Toll-like receptors in liver disease. Adv. Clin. Chem. 59: 155-201. doi: 10.1016/b978-0-12-405211-6.00006-1.
85. Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, Olefsky JM, Brenner DA, Seki E (2010) Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology 139 (1): 323-334.e7. doi: 10.1053/j.gastro.2010.03.052.
86. Szabo G, Petrasek J (2015) Inflammasome activation and function in liver disease. Nat. Rev. Gastroenterol. Hepatol. 12 (7): 387-400. doi: 10.1038/nrgastro.2015.94.
87. Wree A, McGeough MD, Peña CA, Schlattjan M, Li H, Inzaugarat ME, Messer K, Canbay A, Hoffman HM, Feldstein AE (2014) NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J. Mol. Med. (Berl). 92 (10): 1069-1082. doi: 10.1007/s00109-014-1170-1.
88. Sutti S, Jindal A, Locatelli I, Vacchiano M, Gigliotti L, Bozzola C, Albano E (2014) Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology 59 (3): 886-897. doi: 10.1002/hep.26749.
89. Sullivan EM, Fix A, Crouch MJ, Sparagna GC, Zeczycki TN, Brown DA, Shaikh SR (2017) Murine diet-induced obesity remodels cardiac and liver mitochondrial phospholipid acyl chains with differential effects on respiratory enzyme activity. J. Nutr. Biochem. 45: 94-103. doi: 10.1016/j.jnutbio.2017.04.004.
90. Kobayashi T, Kuroda S, Tada M, Houkin K, Iwasaki Y, Abe H (2003) Calcium-induced mitochondrial swelling and cytochrome c release in the brain: its biochemical characteristics and implication in ischemic neuronal injury. Brain Res. 960 (1-2): 62-70. doi: 10.1016/s0006-8993(02)03767-8.
91. Meex RCR, Blaak EE (2021) Mitochondrial Dysfunction is a Key Pathway that Links Saturated Fat Intake to the Development and Progression of NAFLD. Mol. Nutr. Food Res. 65 (1): e1900942. doi: 10.1002/mnfr.201900942.
92. Sell H, Habich C, Eckel J (2012) Adaptive immunity in obesity and insulin resistance. Nat. Rev. Endocrinol. 8 (12): 709-716. doi: 10.1038/nrendo.2012.114.
93. Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, Jankowiak F, Herder C, Carstensen M, Krausch M, Knoefel WT, Schlensak M, Roden M (2015) Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 21 (5): 739-746. doi: 10.1016/j.cmet.2015.04.004.
94. Serviddio G, Bellanti F, Tamborra R, Rollo T, Capitanio N, Romano AD, Sastre J, Vendemiale G, Altomare E (2008) Uncoupling protein-2 (UCP2) induces mitochondrial proton leak and increases susceptibility of non-alcoholic steatohepatitis (NASH) liver to ischaemia-reperfusion injury. Gut 57 (7): 957-965. doi: 10.1136/gut.2007.147496.
95. Cortez-Pinto H, Chatham J, Chacko VP, Arnold C, Rashid A, Diehl AM (1999) Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: a pilot study. JAMA 282 (17): 1659-1664. doi: 10.1001/jama.282.17.1659.
96. Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR (2000) CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J. Clin. Invest. 105 (8):1067-1075. doi: 10.1172/JCI8814.
97. Marí M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A, Enrich C, Fernandez-Checa JC, García-Ruiz C (2006) Mitochondrial free cholesterol loading sensitizes to TNF-α and Fas-mediated steatohepatitis. Cell Metab. 4 (3):185-198. doi: 10.1016/j.cmet.2006.07.006.
98. Świderska M, Maciejczyk M, Zalewska A, Pogorzelska J, Flisiak R, Chabowski A (2019) Oxidative stress biomarkers in the serum and plasma of patients with non-alcoholic fatty liver disease (NAFLD). Can plasma AGE be a marker of NAFLD? Oxidative stress biomarkers in NAFLD patients. Free Radic. Res. 53 (8): 841-850. doi: 10.1080/10715762.2019.1635691.
99. Kim HJ, Lee Y, Fang S, Kim W, Kim HJ, Kim JW (2020) GPx7 ameliorates non-alcoholic steatohepatitis by regulating oxidative stress. BMB Rep. 53 (6): 317-322. doi: 10.5483/BMBRep.2020.53.6.280.
100. Simon J, Nuñez-García M, Fernández-Tussy P, Barbier-Torres L, Fernández-Ramos D, C, Iruzubieta P, Romero-Gomez M, van Liempd S, Castro A, Nogueiras R, Varela-Rey M, Falcón-Pérez JM, Villa E, Crespo J, Lu SC, Mato JM, Aspichueta P, Delgado TC, Martínez-Chantar ML (2020) Targeting Hepatic Glutaminase 1 Ameliorates Non-alcoholic Steatohepatitis by Restoring Very-Low-Density Lipoprotein Triglyceride Assembly. Cell Metab. 31 (3): 605-622.e10. doi: 10.1016/j.cmet.2020.01.013.
101. Milaciu MV, Vesa ȘC, Bocșan IC, Ciumărnean L, Sâmpelean D, Negrean V, Pop RM, Matei DM, Pașca S, Răchișan AL, Buzoianu AD, Acalovschi M (2019) Paraoxonase-1 serum concentration and PON1 gene polymorphisms: relationship with non-alcoholic fatty liver disease. J. Clin. Med. 8 (12): 2200. doi: 10.3390/jcm8122200.
102. Shin SK, Cho HW, Song SE, Song DK (2018) Catalase and nonalcoholic fatty liver disease. Pflugers Arch. 470 (12): 1721-1737. doi: 10.1007/s00424-018-2195-z.
103. Ron D (2002) Translational control in the endoplasmic reticulum stress response. J. Clin. Invest. 110 (10): 1383-1388. doi: 10.1172/JCI16784.
104. Hotamisligil GS (2010) Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140 (6): 900-917. doi: 10.1016/j.cell.2010.02.034.
105. Farrukh MR, Nissar UA, Afnan Q, Rafiq RA, Sharma L, Amin S, Kaiser P, Sharma PR, Tasduq SA (2014) Oxidative stress mediated Ca(2+) release manifests endoplasmic reticulum stress leading to unfolded protein response in UV-B irradiated human skin cells. J. Dermatol. Sci. 75 (1): 24-35. doi: 10.1016/j.jdermsci.2014.03.005.
106. Palomer X, Capdevila-Busquets E, Botteri G, Salvadó L, Barroso E, Davidson MM, Michalik L, Wahli W, Vázquez-Carrera M (2014) PPARβ/δ attenuates palmitate-induced endoplasmic reticulum stress and induces autophagic markers in human cardiac cells. Int. J. Cardiol. 174 (1): 110-118. doi: 10.1016/j.ijcard.2014.03.176.
107. Lebeaupin C, Vallée D, Hazari Y, Hetz C, Chevet E, Bailly-Maitre B (2018) Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 69 (4): 927-947. doi: 10.1016/j.jhep.2018.06.008.
108. Cui JX, Zeng YQ, Wang H, Chen W, Du JF, Chen QM, Hu YX, Yang L (2011) The effects of DGAT1 and DGAT2 mRNA expression on fat deposition in fatty and lean breeds of pig. Livest. sci. 140 (1-3): 292-296.
109. Liu L, Li C, Fu C, Li F (2016) Dietary niacin supplementation suppressed hepatic lipid accumulation in rabbits. Asian-Australas J. Anim. Sci. 29 (12): 1748-1755. doi: 10.5713/ajas.15.0824.
110. Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS (2004) Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306 (5695): 457-461. doi: 10.1126/science.1103160.
111. Salvadó L, Palomer X, Barroso E, Vázquez-Carrera M (2015) Targeting endoplasmic reticulum stress in insulin resistance. Trends Endocrinol. Metab. 26 (8): 438-448. doi: 10.1016/j.tem.2015.05.007.
112. Hatzis G, Ziakas P, Kavantzas N, Triantafyllou A, Sigalas P, Andreadou I, Ioannidis K, Chatzis S, Filis K, Papalampros A, Sigala F (2013) Melatonin attenuates high fat diet-induced fatty liver disease in rats. World J. Hepatol. 5 (4): 160-169. doi: 10.4254/wjh.v5.i4.160.
113. Yu Y, Chen D, Zhao Y, Zhu J, Dong X (2021) Melatonin ameliorates hepatic steatosis by inhibiting NLRP3 inflammasome in db/db mice. Int. J. Immunopathol. Pharmacol. 35: 20587384211036819. doi: 10.1177/20587384211036819.
114. Li Y, Zhang J, Wan J, Liu A, Sun J (2020) Melatonin regulates Aβ production/clearance balance and Aβ neurotoxicity: A potential therapeutic molecule for Alzheimer's disease. Biomed. Pharmacother. 132: 110887. doi: 10.1016/j.biopha.2020.110887.
115. Saha M, Manna K, Das Saha K (2022) Melatonin Suppresses NLRP3 inflammasome activation via TLR4/NF-κB and P2X7R signaling in high-fat diet-induced murine NASH model. J. Inflamm. Res. 15: 3235-3258. doi: 10.2147/JIR.S343236.
116. Joshi A, Upadhyay KK, Vohra A, Shirsath K, Devkar R (2021) Melatonin induces Nrf2-HO-1 reprogramming and corrections in hepatic core clock oscillations in Non-alcoholic fatty liver disease. FASEB J. 35 (9): e21803. doi: 10.1096/fj.202002556RRR.
117. Mansoori A, Salimi Z, Hosseini SA, Hormoznejad R, Jafarirad S, Bahrami M, Asadi M (2020) The effect of melatonin supplementation on liver indices in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized clinical trials. Complement Ther. Med. 52:102398. doi: 10.1016/j.ctim.2020.102398.
118. Stacchiotti A, Grossi I, García-Gómez R, Patel GA, Salvi A, Lavazza A, De Petro G, Monsalve M, Rezzani R (2019) Melatonin effects on non-alcoholic fatty liver disease are related to microRNA-34a-5p/Sirt1 axis and autophagy. Cells 8 (9): 1053. doi: 10.3390/cells8091053.
119. Li DJ, Tong J, Li YH, Meng HB, Ji QX, Zhang GY, Zhu JH, Zhang WJ, Zeng FY, Huang G, Hua X, Shen FM, Wang P (2019) Melatonin safeguards against fatty liver by antagonizing TRAFs-mediated ASK1 deubiquitination and stabilization in a β-arrestin-1 dependent manner. J. Pineal Res. 67 (4): e12611. doi: 10.1111/jpi.12611.
120. Zhou H, Du W, Li Y, Shi C, Hu N, Ma S, Wang W, Ren J (2018) Effects of melatonin on fatty liver disease: The role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy. J. Pineal Res. 64 (1). doi: 10.1111/jpi.12450.
121. Mi Y, Tan D, He Y, Zhou X, Zhou Q, Ji S (2018) Melatonin Modulates lipid Metabolism in HepG2 Cells Cultured in High Concentrations of Oleic Acid: AMPK Pathway Activation may play an Important Role. Cell Biochem. Biophys. 76 (4): 463-470. doi: 10.1007/s12013-018-0859-0.
122. Wongchitrat P, Klosen P, Pannengpetch S, Kitidee K, Govitrapong P, Isarankura-Na-Ayudhya C (2017) High-fat diet-induced plasma protein and liver changes in obese rats can be attenuated by melatonin supplementation. Nutr. Res. 42: 51-63. doi: 10.1016/j.nutres.2017.04.011.
123. Sun H, Wang X, Chen J, Song K, Gusdon AM, Li L, Bu L, Qu S (2016) Melatonin improves non-alcoholic fatty liver disease via MAPK-JNK/P38 signaling in high-fat-diet-induced obese mice. Lipids Health Dis. 15 (1): 202. doi: 10.1186/s12944-016-0370-9.
124. García-Ruiz I, Solís-Muñoz P, Fernández-Moreira D, Grau M, Colina F, Muñoz-Yagüe T, Solís-Herruzo JA (2014) High-fat diet decreases activity of the oxidative phosphorylation complexes and causes nonalcoholic steatohepatitis in mice. Dis. Model Mech. 7 (11): 1287-1296. doi: 10.1242/dmm.016766.
125. Celinski K, Konturek PC, Slomka M, Cichoz-Lach H, Brzozowski T, Konturek SJ, Korolczuk A (2014) Effects of treatment with melatonin and tryptophan on liver enzymes, parameters of fat metabolism and plasma levels of cytokines in patients with non-alcoholic fatty liver disease--14 months follow up. J. Physiol. Pharmacol. 65 (1): 75-82. PMID: 24622832.
126. Zaitone S, Hassan N, El-Orabi N, El-Awady el-S (2011) Pentoxifylline and melatonin in combination with pioglitazone ameliorate experimental non-alcoholic fatty liver disease. Eur. J. Pharmacol. 662 (1-3): 70-77. doi: 10.1016/j.ejphar.2011.04.049.
127. Ríos-Lugo MJ, Cano P, Jiménez-Ortega V, Fernández-Mateos MP, Scacchi PA, Cardinali DP, Esquifino AI (2010) Melatonin effect on plasma adiponectin, leptin, insulin, glucose, triglycerides and cholesterol in normal and high fat-fed rats. J. Pineal Res. 49 (4): 342-348. doi: 10.1111/j.1600-079X.2010.00798.x.
128. Hussein MR, Ahmed OG, Hassan AF, Ahmed MA (2007) Intake of melatonin is associated with amelioration of physiological changes, both metabolic and morphological pathologies associated with obesity: an animal model. Int. J. Exp. Pathol. 88 (1): 19-29. doi: 10.1111/j.1365-2613.2006.00512.x.
129. Bongiorno D, Ceraulo L, Ferrugia M, Filizzola F, Ruggirello A, Liveri VT (2005) Localization and interactions of melatonin in dry cholesterol/lecithin mixed reversed micelles used as cell membrane models. J. Pineal Res. 38 (4): 292-298. doi: 10.1111/j.1600-079X.2005.00211.x.
130. Sener G, Balkan J, Cevikbaş U, Keyer-Uysal M, Uysal M (2004) Melatonin reduces cholesterol accumulation and prooxidant state induced by high cholesterol diet in the plasma, the liver and probably in the aorta of C57BL/6J mice. J. Pineal Res. 36 (3): 212-216. doi: 10.1111/j.1600-079x.2004.00122.x.
131. Prunet-Marcassus B, Desbazeille M, Bros A, Louche K, Delagrange P, Renard P, Casteilla L, Pénicaud L (2003) Melatonin reduces body weight gain in Sprague Dawley rats with diet-induced obesity. Endocrinology 144 (12): 5347-5352. doi: 10.1210/en.2003-0693.
132. Pita ML, Hoyos M, Martin-Lacave I, Osuna C, Fernández-Santos JM, Guerrero JM (2002) Long-term melatonin administration increases polyunsaturated fatty acid percentage in plasma lipids of hypercholesterolemic rats. J. Pineal Res. 32 (3): 179-186. doi: 10.1034/j.1600-079x.2002.1o851.x.
133. Nieminen P, Käkelä R, Mustonen AM, Hyvärinen H, Asikainen J (2001) Exogenous melatonin affects lipids and enzyme activities in mink (Mustela vison) liver. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 128 (2): 203-211. doi: 10.1016/s1532-0456(00)00190-3.
134. Hoyos M, Guerrero JM, Perez-Cano R, Olivan J, Fabiani F, Garcia-Pergañeda A, Osuna C (2000) Serum cholesterol and lipid peroxidation are decreased by melatonin in diet-induced hypercholesterolemic rats. J. Pineal Res. 28 (3): 150-155. doi: 10.1034/j.1600-079x.2001.280304.x.
135. de Souza CAP, Gallo CC, de Camargo LS, de Carvalho PVV, Olesçuck IF, Macedo F, da Cunha FM, Cipolla-Neto J, do Amaral FG (2019) Melatonin multiple effects on brown adipose tissue molecular machinery. J. Pineal Res. 66 (2): e12549. doi: 10.1111/jpi.12549.
136. Pan S, Guo Y, Hong F, Xu P, Zhai Y (2022) Therapeutic potential of melatonin in colorectal cancer: Focus on lipid metabolism and gut microbiota. Biochim. Biophys. Acta Mol. Basis Dis. 1868 (1): 166281. doi: 10.1016/j.bbadis.2021.166281.
137. Yang W, Tang K, Wang Y, Zhang Y, Zan L (2017) Melatonin promotes triacylglycerol accumulation via MT2 receptor during differentiation in bovine intramuscular preadipocytes. Sci. Rep. 7 (1): 15080. doi: 10.1038/s41598-017-12780-y.
138. Guan Q, Wang Z, Cao J, Dong Y, Chen Y (2021) Mechanisms of Melatonin in Obesity: A Review. Int. J. Mol. Sci. 23 (1): 218. doi: 10.3390/ijms23010218.
139. Hussain SA (2007) Effect of melatonin on cholesterol absorption in rats. J. Pineal Res. 42 (3): 267-271. doi: 10.1111/j.1600-079X.2006.00415.x.
140. Chan TY, Tang PL (1995) Effect of melatonin on the maintenance of cholesterol homeostasis in the rat. Endocr. Res. 21 (3): 681-696. doi: 10.1080/07435809509030483.
141. Müller-Wieland D, Behnke B, Koopmann K, Krone W (1994) Melatonin inhibits LDL receptor activity and cholesterol synthesis in freshly isolated human mononuclear leukocytes. Biochem. Biophys. Res. Commun. 203 (1): 416-421. doi: 10.1006/bbrc.1994.2198.
142. Xu Z, You W, Liu J, Wang Y, Shan T (2020) Elucidating the regulatory role of melatonin in brown, white, and beige adipocytes. Adv. Nutr. 11 (2): 447-460. doi: 10.1093/advances/nmz070.
143. Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM (2012) A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481 (7382): 463-468. doi: 10.1038/nature10777.
144. Tung YT, Chiang PC, Chen YL, Chien YW (2020) Effects of melatonin on lipid metabolism and circulating irisin in sprague-dawley rats with diet-induced obesity. Molecules 25 (15): 3329. doi: 10.3390/molecules25153329.
145. Espino J, Pariente JA, Rodríguez AB (2011) Role of melatonin on diabetes-related metabolic disorders. World J. Diabetes 2 (6): 82-91. doi: 10.4239/wjd.v2.i6.82.
146. Tan DX, Manchester LC, Fuentes-Broto L, Paredes SD, Reiter RJ (2011) Significance and application of melatonin in the regulation of brown adipose tissue metabolism: relation to human obesity. Obes Rev. 12 (3): 167-188. doi: 10.1111/j.1467-789X.2010.00756.x.
147. Cinti S (2006) The role of brown adipose tissue in human obesity. Nutr. Metab. Cardiovasc. Dis. 16 (8): 569-574. doi: 10.1016/j.numecd.2006.07.009.
148. Wolden-Hanson T, Mitton DR, McCants RL, Yellon SM, Wilkinson CW, Matsumoto AM, Rasmussen DD (2000) Daily melatonin administration to middle-aged male rats suppresses body weight, intraabdominal adiposity, and plasma leptin and insulin independent of food intake and total body fat. Endocrinology 141 (2): 487-497. doi: 10.1210/endo.141.2.7311.
149. Srinivasan V, Ohta Y, Espino J, Pariente JA, Rodriguez AB, Mohamed M, Zakaria R (2013) Metabolic syndrome, its pathophysiology and the role of melatonin. Recent Pat. Endocr. Metab. Immune Drug Discov. 7 (1): 11-25. PMID: 22946959.
150. Nduhirabandi F, du Toit EF, Lochner A (2012) Melatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities? Acta Physiol. (Oxf). 205 (2): 209-223. doi: 10.1111/j.1748-1716.2012.02410.x.
151. Rodrigues SC, Pantaleão L, Lellis‐Santos C, Veras K, Amaral F, Anhê G, Bordin S (2013) Increased corticosterone levels contribute to glucose intolerance induced by the absence of melatonin. FASEB J. 27: 1161. doi:10.1096/fasebj.27.1_supplement.1161.1.
152. Sun H, Wang X, Chen J, Gusdon AM, Song K, Li L, Qu S (2018) Melatonin treatment improves insulin resistance and pigmentation in obese patients with acanthosis nigricans. Int. J. Endocrinol. 2018:2304746. doi: 10.1155/2018/2304746.
153. Oliveira AC, Andreotti S, Sertie RAL, Campana AB, de Proença ARG, Vasconcelos RP, Oliveira KA, Coelho-de-Souza AN, Donato-Junior J, Lima FB (2018) Combined treatment with melatonin and insulin improves glycemic control, white adipose tissue metabolism and reproductive axis of diabetic male rats. Life Sci. 199: 158-166. doi: 10.1016/j.lfs.2018.02.040.
154. Pourhanifeh MH, Hosseinzadeh A, Dehdashtian E, Hemati K, Mehrzadi S (2020) Melatonin: new insights on its therapeutic properties in diabetic complications. Diabetol. Metab. Syndr. 12: 30. doi: 10.1186/s13098-020-00537-z.
155. Xu L, Bai Q, Rodriguez-Agudo D, Hylemon PB, Heuman DM, Pandak WM, Ren S (2010) Regulation of hepatocyte lipid metabolism and inflammatory response by 25-hydroxycholesterol and 25-hydroxycholesterol-3-sulfate. Lipids 45 (9): 821-832. doi: 10.1007/s11745-010-3451-y.
156. Glass CK, Olefsky JM (2012) Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 15 (5): 635-645. doi: 10.1016/j.cmet.2012.04.001.
157. Sun H, Wang X, Chen J, Song K, Gusdon AM, Li L, Bu L, Qu S (2016) Melatonin improves non-alcoholic fatty liver disease via MAPK-JNK/P38 signaling in high-fat-diet-induced obese mice. Lipids Health Dis. 15 (1): 202. doi: 10.1186/s12944-016-0370-9.
158. Hu S, Yin S, Jiang X, Huang D, Shen G (2009) Melatonin protects against alcoholic liver injury by attenuating oxidative stress, inflammatory response, and apoptosis. Eur. J. Pharmacol. 616 (1-3): 287-292. doi: 10.1016/j.ejphar.2009.06.044.
159. Shaji AV, Kulkarni SK, Agrewala JN (1998) Regulation of secretion of IL-4 and IgG1 isotype by melatonin-stimulated ovalbumin-specific T cells. Clin. Exp. Immunol. 111 (1): 181-185. doi: 10.1046/j.1365-2249.1998.00493.x.
160. Arzt ES, Fernández-Castelo S, Finocchiaro LM, Criscuolo ME, Díaz A, Finkielman S, Nahmod VE (1988) Immunomodulation by indoleamines: serotonin and melatonin action on DNA and interferon-gamma synthesis by human peripheral blood mononuclear cells. J. Clin. Immunol. 8 (6): 513-20. doi: 10.1007/BF00916958.
161. Agil A, Reiter RJ, Jiménez-Aranda A, Ibán-Arias R, Navarro-Alarcón M, Marchal JA, Adem A, Fernández-Vázquez G (2013) Melatonin ameliorates low-grade inflammation and oxidative stress in young Zucker diabetic fatty rats. J. Pineal Res. 54 (4): 381-388. doi: 10.1111/jpi.12012.
162. Ozkanlar S, Kara A, Sengul E, Simsek N, Karadeniz A, Kurt N (2016) Melatonin modulates the immune system response and inflammation in diabetic rats experimentally-induced by alloxan. Horm. Metab. Res. 48 (2): 137-144. doi: 10.1055/s-0035-1548937.
163. Li JH, Yu JP, Yu HG, Xu XM, Yu LL, Liu J, Luo HS (2005) Melatonin reduces inflammatory injury through inhibiting NF-kappaB activation in rats with colitis. Mediators Inflamm. 2005 (4): 185-193. doi: 10.1155/MI.2005.185.
164. Yapislar H, Haciosmanoglu E, Sarioglu T, Degirmencioglu S, Sogut I, Poteser M, Ekmekcioglu C (2022) Anti-inflammatory effects of melatonin in rats with induced type 2 diabetes mellitus. Life (Basel). 12 (4): 574. doi: 10.3390/life12040574.
165. Galano A, Tan DX, Reiter RJ (2013) On the free radical scavenging activities of melatonin's metabolites, AFMK and AMK. J. Pineal Res. 54 (3): 245-257. doi: 10.1111/jpi.12010.
166. Cipolla-Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ (2014) Melatonin, energy metabolism, and obesity: a review. J. Pineal Res. 56 (4): 371-381. doi: 10.1111/jpi.12137.
167. Yoon Y, Krueger EW, Oswald BJ, McNiven MA (2003) The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol. Cell Biol. 23 (15): 5409-5420. doi: 10.1128/MCB.23.15.5409-5420.2003.
168. Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 160 (2):189-200. doi: 10.1083/jcb.200211046.
169. Das N, Mandala A, Naaz S, Giri S, Jain M, Bandyopadhyay D, Reiter RJ, Roy SS (2017) Melatonin protects against lipid-induced mitochondrial dysfunction in hepatocytes and inhibits stellate cell activation during hepatic fibrosis in mice. J. Pineal Res. 62 (4). doi: 10.1111/jpi.12404.
170. Biel TG, Lee S, Flores-Toro JA, Dean JW, Go KL, Lee MH, Law BK, Law ME, Dunn WA Jr, Zendejas I, Behrns KE, Kim JS (2015) Sirtuin 1 suppresses mitochondrial dysfunction of ischemic mouse livers in a mitofusin 2-dependent manner. Cell Death Differ. 23 (2): 279-290. doi: 10.1038/cdd.2015.96.
171. Nakamura S, Takamura T, Matsuzawa-Nagata N, Takayama H, Misu H, Noda H, Nabemoto S, Kurita S, Ota T, Ando H, Miyamoto K, Kaneko S (2009) Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J. Biol. Chem. 284 (22): 14809-14818. doi: 10.1074/jbc.M901488200.
172. Perry RJ, Samuel VT, Petersen KF, Shulman GI (2010) The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 510 (7503): 84-91. doi: 10.1038/nature13478.
173. Tahan V, Atug O, Akin H, Eren F, Tahan G, Tarcin O, Uzun H, Ozdogan O, Tarcin O, Imeryuz N, Ozguner F, Celikel C, Avsar E, Tozun N (2009) Melatonin ameliorates methionine- and choline-deficient diet-induced nonalcoholic steatohepatitis in rats. J. Pineal Res. 46 (4): 401-407. doi: 10.1111/j.1600-079X.2009.00676.x.
174. Koc M, Taysi S, Buyukokuroglu ME, Bakan N (2003) Melatonin protects rat liver against irradiation-induced oxidative injury. J. Radiat. Res. 44 (3): 211-215. doi: 10.1269/jrr.44.211.
175. Konturek SJ, Konturek PC, Brzozowska I, Pawlik M, Sliwowski Z, Cześnikiewicz-Guzik M, Kwiecień S, Brzozowski T, Bubenik GA, Pawlik WW (2007) Localization and biological activities of melatonin in intact and diseased gastrointestinal tract (GIT). J. Physiol. Pharmacol. 58 (3): 381-405. PMID: 17928638.
176. Hong RT, Xu JM, Mei Q (2009) Melatonin ameliorates experimental hepatic fibrosis induced by carbon tetrachloride in rats. World J. Gastroenterol. 15 (12):1452-8. doi: 10.3748/wjg.15.1452.
177. Tahan V, Ozaras R, Canbakan B, Uzun H, Aydin S, Yildirim B, Aytekin H, Ozbay G, Mert A, Senturk H (2004) Melatonin reduces dimethylnitrosamine-induced liver fibrosis in rats. J. Pineal Res. 37 (2): 78-84. doi: 10.1111/j.1600-079X.2004.00137.x.
178. Cichoz-Lach H, Celinski K, Konturek PC, Konturek SJ, Slomka M (2010) The effects of L-tryptophan and melatonin on selected biochemical parameters in patients with steatohepatitis. J. Physiol. Pharmacol. 61 (5): 577-580. PMID: 21081801.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


