Transgenerational effects of maternal circadian melatonin deficiency and melatonin replacement in rats during pregnancy and lactation on the energy metabolism and thermoregulation in the offspring subjected to a high-fat diet
Transgenerational effect of maternal circadian melatonin deficiency on offspring
Abstract
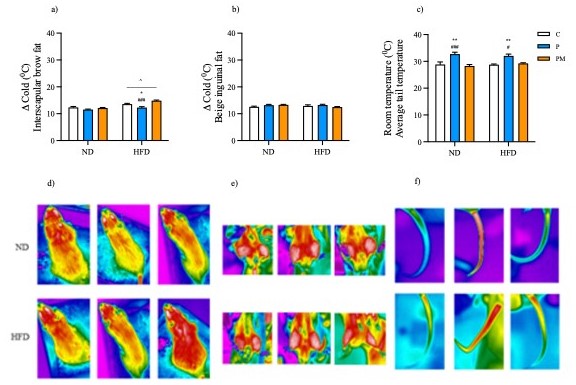
Pineal melatonin participates in the control of numerous biological functions through its immediate effects, which result from its high presence in the systemic circulation during the dark phase of the circadian cycle or, through its prolonged effects, when its level is extremely low during the light phase. At pregnancy, maternal melatonin signals the external photoperiod to the fetus, highlighting its importance not only in synchronizing rhythms, but also in preparing the fetus to adapt the external environments. The fetus and newborns are exclusively dependent on maternal melatonin since their pineal melatonin production only occurs weeks after birth. Thus, maternal hypomelatoninemia stands out as an important factor capable of modulating the physiological systems of their descendants, demonstrating its transgenerational capacity. The present study evaluated the transgenerational influence of maternal melatonin deficiency and replacement during pregnancy on morphometric parameters, thermoregulation and energy metabolism of the offspring submitted to the normal and high-fat diets, respectively. For this, nulliparous Wistar rats at an age of 8 weeks were used and randomized into three groups: CTL (pregnant rats), PINX (pinealectomized pregnant rats), PINX + MEL (pinealectomized pregnant rats submitted to melatonin replacement). After birth, the pups were divided into three groups: (C) pups from control mothers, (P) pups from PINX mothers and (PM) pups from PINX + MEL mothers. One week after weaning, part of the animals was fed a high-fat diet (DH) and rest of them were fed a normal diet (ND) for 12 weeks. Subsequently, the animals were euthanized at ZTs 6 and 18. The results showed that maternal melatonin deficiency disrupted the energy metabolism of the offspring and melatonin replacement normalized the energy metabolism in the offspring submitted to the high-fat diet, enabling them to make functional adaptations such as reduced food consumption and greater thermoregulatory capacity, resulting in reduction in body weight gain white adipose tissue mass.
References
2. Gomez FJ, Raba J, Cerutti S, Silva MF (2012) Monitoring melatonin and its isomer in Vitis vinifera cv. Malbec by UHPLC-MS/MS from grape to bottle. J. Pineal Res. 52: 349-355.
3. Migliori ML, Romanowski A, Simonetta SH, Valdez D, Guido M, Golombek DA. (2012) Daily variation in melatonin synthesis and arylalkylamine N-acetyltransferase activity in the nematode Caenorhabditis elegans. J. Pineal Res. 53: 38-46.
4. Cipolla-Neto J, Amaral FGD. (2018) Melatonin as a hormone: New physiological and clinical insights. Endocr. Rev. 39: 990-1028.
5. Amaral FGD, Andrade-Silva J, Kuwabara WMT, Cipolla-Neto J. (2019) New insights into the function of melatonin and its role in metabolic disturbances. Expert. Rev. Endocrinol. Metab. 14: 293-300.
6. Montilla PL, Túnez IF, Muñoz de Agueda C, Gascón FL, Soria JV. (1998) Protective role of melatonin and retinol palmitate in oxidative stress and hyperlipidemic nephropathy induced by adriamycin in rats. J. Pineal Res. 25: 86-93.
7. Okatani Y, Wakatsuki A, Kaneda C. (2000) Melatonin increases activities of glutathione peroxidase and superoxide dismutase in fetal rat brain. J. Pineal Res. 28: 89-96.
8. Konturek SJ, Konturek PC, Brzozowska I, Pawlik M, Sliwowski Z, Cześnikiewicz-Guzik M, Kwiecień S (2007) Localization and biological activities of melatonin in intact and diseased gastrointestinal tract (GIT). J. Physiol. Pharmacol. 58: 381-405.
9. Leja-Szpak A, Jaworek J, Szklarczyk J, Konturek SJ, Pawlik WW (2007) Melatonin stimulates HSP27 phosphorylation in human pancreatic carcinoma cells (PANC-1). J. Physiol. Pharmacol. 58 Suppl 3: 177-188.
10. Cipolla-Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ. (2014) Melatonin, energy metabolism, and obesity: a review. J. Pineal Res. 56: 371-381.
11. Yellon SM, Longo FD. (1988) Effect of maternal pinealectomy and reverse photoperiod on the circadian melatonin rhythm in the sheep and fetus during the last trimester of pregnancy. Biol. Reprod. 39: 1093-1099.
12. Reiter RJ, Tan DX, Korkmaz A, Rosales-Corral SA (2014) Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum. Reprod. Update 20: 293-307.
13. Gomes PRL, Motta-Teixeira LC, Gallo CC, Carmo Buonfiglio DD, Camargo LS, Quintela T (2021) Maternal pineal melatonin in gestation and lactation physiology, and in fetal development and programming. Gen. Comp. Endocrinol. 300: 113633.
14. Kennaway DJ, Goble FC, Stamp GE (1996) Factors influencing the development of melatonin rhythmicity in humans. J. Clin. Endocrinol. Metab. 81: 1525-1532.
15. Okatani Y, Okamoto K, Hayashi K, Wakatsuki A, Tamura S, Sagara Y (1998) Maternal-fetal transfer of melatonin in pregnant women near term. J. Pineal Res. 25: 129-134.
16. Zhu JL, Hjollund NH, Andersen AM, Olsen J. (2004) Shift work, job stress, and late fetal loss: The national birth cohort in Denmark. J. Occup. Environ. Med. 46: 1144-1149.
17. Croteau A, Marcoux S, Brisson C. (2006) Work activity in pregnancy, preventive measures, and the risk of delivering a small-for-gestational-age infant. Am. J. Public Health 96: 846-855.
18. Abeysena C, Jayawardana P, DE A Seneviratne R. (2009) Maternal sleep deprivation is a risk factor for small for gestational age: a cohort study. Aust. N. Z. J. Obstet. Gynaecol. 49: 382-387.
19. Knutsson A. (2003) Health disorders of shift workers. Occup. Med. (Lond) 53: 103-108.
20. Mendez N, Halabi D, Spichiger C, Salazar ER, Vergara K, Alonso-Vasquez P, Pamela Carmona (2016) Gestational chronodisruption impairs circadian physiology in rat male offspring, increasing the risk of chronic disease. Endocrinology 157: 4654-4668.
21. Torres-Farfan C, Rocco V, Monsó C, Valenzuela FJ, Campino C, Germain A (2006) Maternal melatonin effects on clock gene expression in a nonhuman primate fetus. Endocrinology 147: 4618-4626.
22. Bellavía SL, Carpentieri AR, Vaqué AM, Macchione AF, Vermouth NT (2006) Pup circadian rhythm entrainment--effect of maternal ganglionectomy or pinealectomy. Physiol. Behav. 89: 342-349.
23. Kennaway DJ, Stamp GE, Goble FC (1992) Development of melatonin production in infants and the impact of prematurity. J. Clin. Endocrinol. Metab. 75: 367-369.
24. Rivkees SA (1997) Developing circadian rhythmicity. Basic and clinical aspects. Pediatr. Clin. North Am. 44: 467-487.
25. Sheridan MN, Rollag MD (1983) Development and melatonin content of the deep pineal gland in the Syrian hamster. Am. J. Anat. 168: 145-156.
26. Lau C, Rogers JM (2004) Embryonic and fetal programming of physiological disorders in adulthood. Birth Defects Res. C. Embryo Today 72: 300-312.
27. Drake AJ, Walker BR, Seckl JR (2005) Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288: R34-38.
28. Rosenfeld A, Weller A (2012) Behavioral effects of environmental enrichment during gestation in WKY and Wistar rats. Behav. Brain Res. 233: 245-255.
29. Suzuki K (2018) The developing world of DOHaD. J. Dev. Orig. Health Dis. 9: 266-269.
30. Barker DJ. (2007) The origins of the developmental origins theory. J. Intern. Med. 261: 412-417.
31. Nehme PA, Amaral FG, Middleton B, Lowden A, Marqueze E, França-Junior I (2019) Melatonin profiles during the third trimester of pregnancy and health status in the offspring among day and night workers: A case series. Neurobiol. Sleep Circadian Rhythms 6: 70-76.
32. Nehme PA, Amaral F, Lowden A, Skene DJ, Cipolla-Neto J, Moreno CRC. (2019) Reduced melatonin synthesis in pregnant night workers: Metabolic implications for offspring. Med. Hypotheses 132: 109353.
33. Ferreira DS, Amaral FG, Mesquita CC, Barbosa APL, Lellis-Santos C, Turati AO. (2012) Maternal melatonin programs the daily pattern of energy metabolism in adult offspring. Plos One 7 (6): e38795.
34. Motta-Teixeira LC, Machado-Nils AV, Battagello DS, Diniz GB, Andrade-Silva J, Silva Jr J. (2018) The absence of maternal pineal melatonin rhythm during pregnancy and lactation impairs offspring physical growth, neurodevelopment, and behavior. Horm. Behav. 105: 146-156.
35. Diaz B, Blázquez E. (1986) Effect of pinealectomy on plasma glucose, insulin and glucagon levels in the rat. Horm. Metab. Res. 18: 225-229.
36. Anhê GF, Caperuto LC, Pereira-Da-Silva M, Souza LC, Hirata AE, Velloso LA (2004) In vivo activation of insulin receptor tyrosine kinase by melatonin in the rat hypothalamus. J. Neurochem. 90: 559-566.
37. Lima FB, Machado UF, Bartol I, Seraphim PM, Sumida DH, Moraes SM (1998) Pinealectomy causes glucose intolerance and decreases adipose cell responsiveness to insulin in rats. Am. J. Physiol. 275: E934-941.
38. Ghosh G, De K, Maity S, Bandyopadhyay D, Bhattacharya S, Reiter RJ (2007) Melatonin protects against oxidative damage and restores expression of GLUT4 gene in the hyperthyroid rat heart. J. Pineal Res. 42: 71-82.
39. Picinato MC, Hirata AE, Cipolla-Neto J, Curi R, Carvalho CRO, Anhê GF (2008) Activation of insulin and IGF-1 signaling pathways by melatonin through MT1 receptor in isolated rat pancreatic islets. J. Pineal Res. 44: 88-94.
40. Armstrong SM. (1989) Melatonin and circadian control in mammals. Experientia 45: 932-938 (1989).
41. Rosenwald M, Wolfrum C. (2014) The origin and definition of brite versus white and classical brown adipocytes. Adipocyte 3: 4-9.
42. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425-432.
43. Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. (1995) Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Invet. 95: 2409-2415.
44. Mössenböck K, Vegiopoulos A, Rose AJ, Sijmonsma TP, Herzig S, Schafmeier T. (2014) Browning of white adipose tissue uncouples glucose uptake from insulin signaling. PLoS One 9: e110428.
45. Cannon B, Nedergaard B. (2004) Brown adipose tissue: function and physiological significance. Physiol. Rev. 84: 277-359.
46. Bae J, Ricciardi CJ, Esposito D, Komarnytsky S, Hu P, Curry BJ (2014) Activation of pattern recognition receptors in brown adipocytes induces inflammation and suppresses uncoupling protein 1 expression and mitochondrial respiration. Am. J. Physiol. Cell. Physiol. 306: C918-930.
47. Gallo CC, Nishino FA, Amaral FG, Cipolla-Neto J. (2002) Pinealectomy in rats. Methods Mol. Biol. 2550: 45-51.
48. Nakamura Y, Tamura H, Kashida S, Takayama H, Yamagata Y, Karube A (2001) Changes of serum melatonin level and its relationship to feto-placental unit during pregnancy. J. Pineal Res. 30: 29-33.
49. Tamura H, Takayama H, Nakamura Y, Reiter RJ, Sugino N. (2008) Fetal/placental regulation of maternal melatonin in rats. J. Pineal Res. 44: 335-340.
50. El-Deen RM, Heeba GH, Abdel-Latif RG, Khalifa MMA. (2020) Comparative effectiveness of phosphodiesterase 3, 4, and 5 inhibitors in amelioration of high-fat diet-induced nonalcoholic fatty liver in rats. Fundam. Clin. Pharmacol. 34: 353-364.
51. Al Nebaihi HM, Batran RA, Ussher JR, Maayah ZH, El-Kadi AOS, Brocks DR. (2020) Dietary-induced obesity, hepatic cytochrome P450, and lidocaine metabolism: comparative effects of high-fat diets in mice and rats and reversibility of effects with normalization of diet. J. Pharm. Sci. 109: 1199-1210.
52. Small L, Brandon AE, Turner N, Cooney GJ. (2018) Modeling insulin resistance in rodents by alterations in diet: what have high-fat and high-calorie diets revealed? Am. J. Physiol. Endocrinol. Metab. 314: E251-E265.
53. Hrushesky WJ. (2005) Molecular biology of circadian rhythms. Clin. Chem. 51: 280-281.
54. Romanovsky AA, Ivanov AI, Shimansky YP. (2002) Selected contribution: ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J. Appl. Physiol. 92: 2667-2679.
55. Meyer CW, Ootsuka Y, Romanovsky AA. (2017) Body temperature measurements for metabolic phenotyping in mice. Front. Physiol. 8: 520.
56. Mendes C, Gomes G, Belpiede LT, Buonfiglio DC, Motta-Teixeira LC, Amaral FG (2021) The effects of melatonin daily supplementation to aged rats on the ability to withstand cold, thermoregulation and body weight. Life Sci. 265: 118769.
57. Vandesompele J, Preter KD, Pattyn F, Poppe B, Roy NV, Paepe ND (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3: RESEARCH0034.
58. Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248-254.
59. Spinazzi M, Casarin A, Pertegato V, Salviati L, Angelini C. (2012) Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat. Protoc. 7: 1235-1246.
60. Buonfiglio D, Parthimos R, Dantas R, Cerqueira Silva R, Gomes G, Andrade-Silva J (2018) Melatonin absence leads to long-term leptin resistance and overweight in rats. Front. Endocrinol. (Lausanne) 9, 122.
61. Klein DC. (1972) Evidence for the placental transfer of 3 H-acetyl-melatonin. Nat. New Biol. 237: 117-118.
62. Vermouth NT, Carriazo CS, Gallará RV, Carpentieri AR, Bellavía SL (1995) Maternal coordination of the daily rhythm of malate dehydrogenase activity in testes from young rats: effect of maternal sympathetic denervation of the pineal gland and administration of melatonin. Chronobiol. Int. 12: 8-18.
63. Schenker S, Yang Y, Perez A, Acuff RV, Papas AM, Henderson G (1998) Antioxidant transport by the human placenta. Clin. Nutr. 17: 159-167.
64. Wilkinson D, Shepherd E, Wallace EM. (2016) Melatonin for women in pregnancy for neuroprotection of the fetus. Cochrane Database Syst. Rev. 3: CD010527.
65. de Farias TSM, Oliveira AC, Andreotti S, Amaral FG, Chimin P, Proença ARA (2015) Pinealectomy interferes with the circadian clock genes expression in white adipose tissue. J. Pineal Res. 58: 251-261.
66. Chiefari E, Arcidiacono B, Foti D, Brunetti A (2017) Gestational diabetes mellitus: an updated overview. J. Endocrinol. Invest. 40: 899-909.
67. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study Cooperative Research Group. (2010) Hyperglycemia and adverse pregnancy outcome (HAPO) study: preeclampsia. Am. J. Obstet. Gynecol. 202: 255.e251-257.
68. Tan DX, Manchester LC, Fuentes-Broto L,. Paredes SD, Reiter RJ. (2011) Significance and application of melatonin in the regulation of brown adipose tissue metabolism: relation to human obesity. Obes. Rev. 12: 167-188.
69. Alonso-Vale MIC, Anhê GF, Borges-Silva CN, Andreotti S, Peres SB, Cipolla-Neto J (2004) Pinealectomy alters adipose tissue adaptability to fasting in rats. Metabolism 53: 500-506.
70. Wolden-Hanson T, Mitton DR, McCants RL, Yellon SM, Wilkinson CW, Matsumoto AM (2000) Daily melatonin administration to middle-aged male rats suppresses body weight, intraabdominal adiposity, and plasma leptin and insulin independent of food intake and total body fat. Endocrinology 141: 487-497.
71. Amaral FG, Turati AO, Barone M, Scialfa JH, Buonfiglio DC, Peres R (2014) Melatonin synthesis impairment as a new deleterious outcome of diabetes-derived hyperglycemia. J. Pineal Res. 57: 67-79.
72. James DE, Burleigh KM, Kraegen EW (1985) Time dependence of insulin action in muscle and adipose tissue in the rat in vivo. An increasing response in adipose tissue with time. Diabetes 34: 1049-1054.
73. Caro JF. (1991) Clinical review 26: Insulin resistance in obese and nonobese man. J. Clin. Endocrinol. Metab. 73: 691-695.
74. Wu YH, Zhou JN, Balesar R, Unmehopa U, Bao A, Jockers R (2006) Distribution of MT1 melatonin receptor immunoreactivity in the human hypothalamus and pituitary gland: colocalization of MT1 with vasopressin, oxytocin, and corticotropin-releasing hormone. J. Comp. Neurol. 499: 897-910.
75. Mårtensson LG, Andersson RG, Berg G. (1996) Melatonin together with noradrenaline augments contractions of human myometrium. Eur. J. Pharmacol. 316: 273-275.
76. Aarseth JJ, Nordøy ES,. Stokkan KA. (2001) Melatonin potentiates the vasoconstrictive effect of noradrenaline in renal artery from newborn hooded seals (Cystophora cristata) and harp seals (Phoca groenlandica). J. Comp. Physiol. B 171: 491-496.
77. Bamshad M, Song CK, Bartness TJ. (1999) CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am. J. Physiol. 276: R1569-1578.
78. Bartness TJ, Demas GE, Song CK. (2002) Seasonal changes in adiposity: the roles of the photoperiod, melatonin and other hormones, and sympathetic nervous system. Exp. Biol. Med. (Maywood) 227: 363-376.
79. Seron-Ferre M, Reynolds H, Mendez NA, Mondaca M, Valenzuela F, Ebensperger R (2014) Impact of maternal melatonin suppression on amount and functionality of brown adipose tissue (BAT) in the newborn sheep. Front. Endocrinol. (Lausanne) 5: 232.
80. Liang X, Yang Q, Zhang L, Maricelli JW, Rodgers BD, Zhu MJ (2016) Maternal high-fat diet during lactation impairs thermogenic function of brown adipose tissue in offspring mice. Sci. Rep. 6: 34345.
81. Kalinovich AV, Jong JM, Cannon B, Nedergaard J. (2017) UCP1 in adipose tissues: two steps to full browning. Biochimie 134: 127-137.
82. Schulz TJ, Huang P, Huang TL, Xue R, McDougall LE, Townsend KL (2013) Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature 495: 379-383.
83. Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC (2012) A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481: 463-468.
84. Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH. (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150: 366-376.
85. Kajimura S, Seale P, Spiegelman BM. (2010) Transcriptional control of brown fat development. Cell Metab. 11: 257-262.
86. Salagre D, Chayah M, Carballo AM, Oliveras-López MJ, Munoz-Hoyos A, Navarro-Alarcón M (2022) Melatonin induces fat browning by transdifferentiation of white adipocytes and de novo differentiation of mesenchymal stem cells. Food Funct. 13: 3760-3775.
87. Acuna-Castroviejo D, Escames G, Rodriguez MI, Lopez LC. (2007) Melatonin role in the mitochondrial function. Front. Biosci. 12: 947-963.
88. Reiter RJ, Tan DX, Qi W, Manchester LC, Karbownik M, Calvo JR. (2000) Pharmacology and physiology of melatonin in the reduction of oxidative stress in vivo. Biol. Signals Recept. 9: 160-171.
89. Reiter RJ, Tan DX, Manchester LC, Qi W (2001) Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence. Cell Biochem. Biophys. 34: 237-256.
90. Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM (2002) Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med.. Chem 2: 181-197.
91. Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. (2007) One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 42: 28-42.
92. Acuña-Castroviejo D, Escames G, López LC, Hitos AB, León J. (2005) Melatonin and nitric oxide: two required antagonists for mitochondrial homeostasis. Endocrine 27: 159-168.
93. López LC, Escames G, Tapias V, Utrilla P, León J, Acuña-Castroviejo D (2006) Identification of an inducible nitric oxide synthase in diaphragm mitochondria from septic mice: its relation with mitochondrial dysfunction and prevention by melatonin. Int. J. Biochem. Cell. Biol. 38: 267-278.
94. Rodríguez MI, Escames G, López LC, López A, García JA, Ortiz F (2008) Improved mitochondrial function and increased life span after chronic melatonin treatment in senescent prone mice. Exp. Gerontol. 43: 749-756.
95. Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T (2009) Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360: 1518-1525.
96. Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. (2009) UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell. Metab. 9: 203-209.
97. Stock MJ (1989) Thermogenesis and brown fat: relevance to human obesity. Infusionstherapie 16: 282-284.
98. Rothwell NJ, Stock MJ. (1981) Influence of noradrenaline on blood flow to brown adipose tissue in rats exhibiting diet-induced thermogenesis. Pflugers Arch. 389: 237-242.
99. Seals DR, Bell C. (2004) Chronic sympathetic activation: consequence and cause of age-associated obesity? Diabetes 53: 276-284.
100. Fromme T, Klingenspor M. (2011) Uncoupling protein 1 expression and high-fat diets. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300: R1-8.
101. Lockie SH, Stefanidis A, Oldfield BJ, Perez-Tilve D. (2013) Brown adipose tissue thermogenesis in the resistance to and reversal of obesity: A potential new mechanism contributing to the metabolic benefits of proglucagon-derived peptides. Adipocyte 2: 196-200.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


