The pleiotropic role of melatonin against chromium-induced cardiovascular infirmities: a mechanistic insight
Melatonin protects chromium induced myocardial damages
Abstract
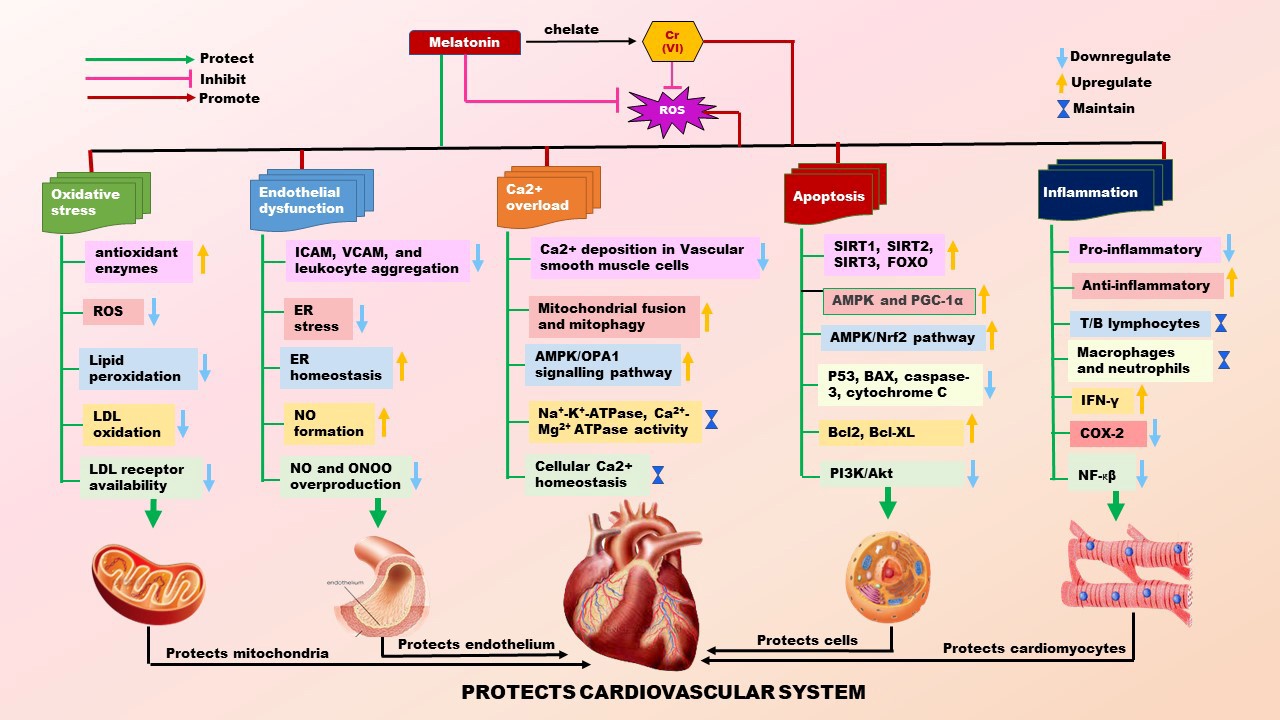
Currently, cardiovascular diseases are still the number one killer in the world. These include hypertension, coronary heart disease, ischemic heart disease, myocardial infarction, congestive heart failure, cardiac arrhythmias, etc. One of the risk factors for cardiovascular diseases is environmental heavy metal pollution which makes the victims more vulnerable to sudden cardiac death. Chromium (Cr) is one of the metals. Cr(VI) is the most hazardous one among its variants. It is readily across the plasma membrane to cause oxidative damage to intracellular molecules including LDL, proteins, and DNA; therefore, promotes endothelial dysfunction and Ca2+ overload in the heart. As to its molecular mechanism, Cr(VI) downregulates the expressions of SIRTUINS, FOXOs, PGC-1α, and AMPK and upregulates the P53, Akt, and NF-κβ, causing alteration in metabolic pathways, inhibiting mitochondrial biogenesis, inducing autophagy and apoptosis. In addition, Cr(VI) alters the expressions of Th1 cytokines (IL-1β, IL-2, IL-12, TNF-α, and IFN-γ) as well as Th2 cytokines (IL-4, IL-5, and IL-10) to induce myocardial inflammation. Melatonin, a potent antioxidant, and an efficient metal chelator can neutralize almost all the alterations caused by Cr(VI). Thus, melatonin can be a selected molecule to protect against Cr(VI)-induced cardiovascular toxicity. This review highlights the etiology of Cr(VI) associated heart diseases and the potentiality of melatonin to prevent Cr(VI)-mediated cardiac oxidative stress, apoptosis and inflammation.
References
2. Tanwar V, Katapadi A, Adelstein JM, Grimmer JA, Wold LE (2018) Cardiac pathophysiology in response to environmental stress: a current review. Curr. Opin. Physiol. 1: 198–205. DOI: 10.1016/j.cophys.2017.11.005.
3. Buhari O, Dayyab FM, Igbinoba O, Atanda A, Medhane F, Faillace RT (2020) The association between heavy metal and serum cholesterol levels in the US population: National Health and Nutrition Examination Survey 2009–2012. Hum. Exp. Toxicol. 39 (3): 355–364. DOI: 10.1177/0960327119889654.
4. Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M (2021) Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 12 (12): 1–19. DOI: 10.3389/fphar.2021.643972.
5. Tsutsui H, Kinugawa S, Matsushima S (2011) Oxidative stress and heart failure. Am. J. Physiol. - Hear. Circ. Physiol. 301 (6): 2181–2190. DOI: 10.1152/ajpheart.00554.2011.
6. Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy Metal Toxicity and the Environment. Mol. Clin. Environ. Toxicol. 101: 133–164. DOI: 10.1007/978-3-7643-8340-4.
7. Ghosh P, Dey T, Chattopadhyay A, Bandyopadhyay D (2021) An insight into the ameliorative effects of melatonin against chromium induced oxidative stress and DNA damage: a review. Melatonin Res. 4 (3): 377–407. DOI: 10.32794/MR112500101.
8. Ali A, Ma Y, Reynolds J, Wise JP, Inzucchi SE, Katz DL (2011) Chromiun effects on glucose tolerance and insulin sensitivity in persons at risk for diabetes mellitus. Endocr. Pract. 17 (1): 16–25. DOI: 10.4158/EP10131.OR.
9. Sahin K, Onderci M, Tuzcu M, Ustundag B, Cikim G, Ozercan IH, Sriramoju V, Juturu V, Komorowski JR (2007) Effect of chromium on carbohydrate and lipid metabolism in a rat model of type 2 diabetes mellitus: the fat-fed, streptozotocin-treated rat. Metabolism 56 (9): 1233–1240. DOI: 10.1016/j.metabol.2007.04.021.
10. Hua Y, Clark S RJ and NS (2012) Molecular Mechanisms of Chromium in Alleviating Insulin Resistance. J. Nutr. Biochem. 23 (4): 313–319. DOI: 10.1016/j.jnutbio.2011.11.001.Molecular.
11. Sandra S. Wise JPW (2012) Chromium and genomic stability. Mutat. Res. 733 (1–2): 78–82. DOI: 10.1016/j.mrfmmm.2011.12.002.
12. Qi W, Reiter RJ, Tan DX, Garcia JJ, Manchester LC, Karbownik M, Calvo JR (2000) Chromium(III)-induced 8-hydroxydeoxyguanosine in DNA and its reduction by antioxidants: Comparative effects of melatonin, ascorbate, and vitamin E. Environ. Health Perspect. 108 (5): 399–403. DOI: 10.1289/ehp.00108399.
13. Bagchi D, Bagchi M, Stohs SJ (2001) Chromium (VI)-induced oxidative stress, apoptotic cell death and modulation of p53 tumor suppressor gene. Mol. Cell. Biochem. 222 (1–2): 149–158. DOI: 10.1023/A:1017958028256.
14. Bagchi D, Balmoori J, Bagchi M, Ye X, Williams CB, Stohs SJ (2000) Role of P53 tumor suppressor gene in the toxicity of TCDD, endrin, naphthalene, and chromium (VI) in liver and brain tissues of mice. Free Radic. Biol. Med. 28 (6): 895–903. DOI: 10.1016/s0891-5849(00)00173-8.
15. Fang Z, Zhao M, Zhen H, Chen L, Shi P, Huang Z (2014) Genotoxicity of tri- and hexavalent chromium compounds in vivo and their modes of action on DNA damage in vitro. PLoS One 9 (8): 1–9. DOI: 10.1371/journal.pone.0103194.
16. Yang D, Yang Q, Fu N, Li S, Han B, Liu Y, Tang Y, Guo X, Lv Z, Zhang Z (2021) Hexavalent chromium induced heart dysfunction via Sesn2-mediated impairment of mitochondrial function and energy supply. Chemosphere 264 (128547): 1–10. DOI: 10.1016/j.chemosphere.2020.128547.
17. Abraham AS, Sonnenblick M, Eni M, Shemesh O, Batt AP (1980) Serum chromium in patients with recent and old myocardial infarction. Am. Heart J. 99 (5): 604–606. DOI: 10.1016/0002-8703(80)90734-6.
18. Gutiérrez-Bedmar M, Martínez-González MÁ, Muñoz-Bravo C, Ruiz-Canela M, Mariscal A, Salas-Salvadó J et al. (2017) Chromium exposure and risk of cardiovascular disease in high cardiovascular risk subjects: Nested case-control study in the prevention with mediterranean diet (PREDIMED) study. Circ. J. 81 (8): 1183–1190. DOI: 10.1253/circj.CJ-17-0032.
19. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP et al. (2019) Heart disease and stroke statistics-2019 update: A report from the american heart association. Circulation 139 (10): e56–e528. DOI: 10.1161/CIR.0000000000000659.
20. Sharman EH, Bondy SC (2016) Melatonin: A safe nutraceutical and clinical agent. Nutraceuticals: efficacy, safety and toxicity. Elsevier Inc.; 2016. 501–509 p. DOI: 10.1016/B978-0-12-802147-7.00036-X.
21. Domínguez-Rodríguez A, Abreu-González P, Báez-Ferrer N, Reiter RJ, Avanzas P, Hernández-Vaquero D (2021) Melatonin and cardioprotection in humans: A systematic review and meta-Analysis of randomized controlled trials. Front. Cardiovasc. Med. 12 (8): 1–6. DOI: 10.3389/fcvm.2021.635083.
22. Tan DX, Hardeland R, Manchester LC, Korkmaz A, Ma S, Rosales-Corral S, Reiter RJ (2012) Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 63 (2): 577–597. DOI: 10.1093/jxb/err256.
23. Jiki Z, Lecour S, Nduhirabandi F (2018) Cardiovascular benefits of dietary melatonin: A myth or a reality? Front. Physiol. 9 (528): 1–17. DOI: 10.3389/fphys.2018.00528.
24. Yang Y, Sun Y, Yi W, Li Y, Fan C, Xin Z, Jiang S, Di S, Qu Y, Reiter RJ, Yi D (2014) A review of melatonin as a suitable antioxidant against myocardial ischemia-reperfusion injury and clinical heart diseases. J. Pineal Res. 57 (4): 357–366. DOI: 10.1111/jpi.12175.
25. Koziróg M, Poliwczak AR, Duchnowicz P, Koter-Michalak M, Sikora J, Broncel M (2011) Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J. Pineal Res. 50 (3): 261–266. DOI: 10.1111/j.1600-079X.2010.00835.x.
26. Dominguez-Rodriguez A, Abreu-Gonzalez P, Reiter RJ (2014) The potential usefulness of serum melatonin level to predict heart failure in patients with hypertensive cardiomyopathy. Int. J. Cardiol. 174 (2): 415–417. DOI: 10.1016/j.ijcard.2014.04.044.
27. Altun A, Yaprak M, Aktoz M, Vardar A, Betul UA, Ozbay G (2002) Impaired nocturnal synthesis of melatonin in patients with cardiac syndrome X. Neurosci. Lett. 327 (2): 143–145. DOI: 10.1016/S0304-3940(02)00368-3.
28. Domínguez-Rodríguez A, Abreu-González P, García MJ, Sanchez J, Marrero F, De Armas-Trujillo D (2002) Decreased nocturnal melatonin levels during acute myocardial infarction. J. Pineal Res. 33 (4): 248–252. DOI: 10.1034/j.1600-079X.2002.02938.x.
29. Girotti L, Lago M, Ianovsky O, Elizari M V., Dini A, Pérez Lloret S, Albornoz LE, Cardinali DP (2003) Low Urinary 6-Sulfatoxymelatonin Levels in Patients with Severe Congestive Heart Failure. Endocrine 22 (3): 245–248. DOI: 10.1385/ENDO:22:3:245.
30. Dzida G, Prystupa A, Lachowska-Kotowska P, Kardas T, Kamieński P, Kimak E, Hałabiś M, Kiciński P (2013) Alteration in diurnal and nocturnal melatonin serum level in patients with chronic heart failure. Ann. Agric. Environ. Med. 20 (4): 745–748.
31. Siegel D, Black DM, Seeley DG, Hulley SB (1992) Circadian Variation in Ventricular arrhythmias in hypertensive men. Am. J. Cardiol. 69 (4): 344–347. DOI: 10.1016/0002-9149(92)90231-m.
32. Savopoulos C, Ziakas A, Hatzitolios A, Delivoria C, Kounanis A, Mylonas S, Tsougas M, Psaroulis D (2006) Circadian rhythm in sudden cardiac death: A retrospective study of 2,665 cases. Angiology 57 (2): 197–204. DOI: 10.1177/000331970605700210.
33. Sun H, Brocato J, Costa M (2015) Oral chromium exposure and toxicity. Curr. Env. Heal. Rep. 2 (3): 295–303. DOI: 10.1007/s40572-015-0054-z.
34. Simonoff M (1984) Chromium deficiency and cardiovascular risk. Cardiovasc. Res. 18 (10): 591–596. DOI: 10.1093/CVR/18.10.591.
35. Nickens KP, Patierno SR, Ceryak S (2010) Chromium genotoxicity: A double-edged sword. Chem. Biol. Interact. 188 (2): 276–288. DOI: 10.1016/j.cbi.2010.04.018.
36. Oliveira H (2012) Chromium as an environmental pollutant: Insights on induced plant toxicity. J. Bot. 2012 : 2090–2120. DOI: 10.1155/2012/375843.
37. Liang J, Huang X, Yan J, Li Y, Zhao Z, Liu Y, Ye J, Wei Y (2021) A review of the formation of Cr(VI) via Cr(III) oxidation in soils and groundwater. Sci. Total Environ. 774 (145762): 1–13. DOI: 10.1016/J.SCITOTENV.2021.145762.
38. Apte AD, Tare V, Bose P (2006) Extent of oxidation of Cr(III) to Cr(VI) under various conditions pertaining to natural environment. J. Hazard. Mater. 128 (2–3): 164–174. DOI: 10.1016/J.JHAZMAT.2005.07.057.
39. Wilbur S, Abadin H, Fay M, Yu D, Tencza B, Ingerman L, Klotzbach J, James S (2012) Toxicological Profile for Chromium. Agency for Toxic Substances and Disease Registry (US) (September): 1-9. www.atsdr.cdc.gov.
40. International Agency for Research on Cancer 2012. (2012) Arsenic, Metals, Fibres, and Dusts. Vol. 1989, IARC Monographs: Arsenic, Metals, Fibres, and Dusts in a Review of Human Carcinogens. 2012. 147–168 p.
41. Wayne W. Campbell , Lyndon J.O Joseph, Richard A. Anderson, Stephen L. Davey JH and WJE (2002) Effects of resistive training and chromium picolinate on body composition and skeletal muscle size in older women. Int. J. Sport Nutr. Exerc. Metab. 12 (2): 125–135. DOI: 10.1123/ijsnem.12.2.125.
42. Anderson RA (1998) Chromium, glucose intolerance and diabetes. J. Am. Coll. Nutr. 17 (6): 548–555. DOI: 10.1080/07315724.1998.10718802.
43. Schwarz K, Mertz W (1959) Chromium(III) and the glucose tolerance factor. Arch. Biochem. Biophys. 85 (1): 292–295. DOI: 10.1016/0003-9861(59)90479-5.
44. Jeejeebhoy KN, Chu RC, Marliss EB, Greenberg GR, Bruce-Robertson A (1977) Chromium deficiency, glucose intolerance, and neuropathy reversed by chromium supplementation, in a patient receiving long-term total parenteral nutrition. Am. J. Clin. Nutr. 30 (4): 531–538. DOI: 10.1093/AJCN/30.4.531.
45. Ngala RA, Awe MA, Nsiah P (2018) The effects of plasma chromium on lipid profile, glucose metabolism and cardiovascular risk in type 2 diabetes mellitus. A case - control study. PLoS One 13 (7): 1–11. DOI: 10.1371/journal.pone.0197977.
46. Scherer T, O’Hare J, Diggs-Andrews K, Schweiger M, Cheng B, Lindtner C, Zielinski E, Vempati P, Su K, Dighe S, Milsom T, Puchowicz M, Scheja L, Zechner R, Fisher SJ, Previs SF, Buettner C (2011) Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell Metab. 13 (2): 183–194. DOI: 10.1016/j.cmet.2011.01.008.
47. Duvillard L, Florentin E, Lizard G, Petit JM, Galland F, Monier S, Gambert P, Vergès B (2003) Cell surface expression of LDL receptor is decreased in type 2 diabetic patients and is normalized by insulin therapy. Diabetes Care 26 (5): 1540–1544. DOI: 10.2337/DIACARE.26.5.1540.
48. Lapenna D, Ciofani G (2020) Chromium and human low-density lipoprotein oxidation. J. Trace Elem. Med. Biol. 59 (126411): 1–29. DOI: 10.1016/J.JTEMB.2019.126411.
49. Abraham AS, Sonnenblick M, Eini M, Shemesh O, Batt AP (1980) The effect of chromium on established atherosclerotic plaques in rabbits. Am. J. Clin. Nutr. 33 (11): 2294–2298. DOI: 10.1093/AJCN/33.11.2294.
50. Uusitupa MI, Kumpulainen JT, Voutilainen E, Hersio K, Sarlund H, Pyörälä KP, Koivistoinen PE, Lehto JT (1983) Effect of Inorganic chromium supplementation on glucose tolerance, insulin response, and serum lipids in noninsulin-dependent diabetics. Am. J. Clin. Nutr. 38 (3): 404–410. DOI: 10.1093/ajcn/38.3.404.
51. Krzysik M, Grajeta H, Prescha A, Weber R (2011) Effect of cellulose, pectin and chromium(III) on lipid and carbohydrate metabolism in rats. J. Trace Elem. Med. Biol. 25 (2): 97–102. DOI: 10.1016/J.JTEMB.2011.01.003.
52. Swaroop A, Bagchi M, Preuss HG, Zafra-Stone S, Ahmad T, Bagchi D 2019. (2019) Benefits of chromium(III) complexes in animal and human health. In: The Nutritional Biochemistry of Chromium (III). Elsevier B.V.; 2019. p. 251–278. DOI: 10.1016/b978-0-444-64121-2.00008-8.
53. Barrett-Connor E (2003) Diabetes and heart disease. Diabetes Care 26 (10): 2947–2958. DOI: 10.2337/DIACARE.26.10.2947.
54. Haffner SM (2009) Coronary heart disease in patients with diabetes. N. Engl. J. Med. 342 (14): 1040–1042. DOI: 10.1056/NEJM200004063421408.
55. Wang ZQ, Qin J, Martin J, Zhang XH, Sereda O, Anderson RA, Pinsonat P, Cefalu WT (2007) Phenotype of subjects with type 2 diabetes mellitus may determine clinical response to chromium supplementation. Metabolism 56 (12): 1652–1655. DOI: 10.1016/j.metabol.2007.07.007.
56. Pattar GR, Tackett L, Liu P, Elmendorf JS (2006) Chromium picolinate positively influences the glucose transporter system via affecting cholesterol homeostasis in adipocytes cultured under hyperglycemic diabetic conditions. Mutat. Res. - Genet. Toxicol. Environ. Mutagen. 610 (1–2): 93–100. DOI: 10.1016/j.mrgentox.2006.06.018.
57. Raja NS, Nair BU (2008) Chromium(III) complexes inhibit transcription factors binding to DNA and associated gene expression. Toxicology 251 (1–3): 61–65. DOI: 10.1016/j.tox.2008.07.052.
58. Das J, Sarkar A, Sil PC (2015) Hexavalent chromium induces apoptosis in human liver (HepG2) cells via redox imbalance. Toxicol. Reports 2: 600–608. DOI: 10.1016/J.TOXREP.2015.03.013.
59. Zhong X, da Silveira e Sá R de C, Zhong C (2017) Mitochondrial biogenesis in response to chromium (VI) toxicity in human liver cells. Int. J. Mol. Sci. 18 (9): 1–17. DOI: 10.3390/IJMS18091877.
60. Beaver LM, Stemmy EJ, Schwartz AM, Damsker JM, Constant SL, Ceryak SM, Patierno SR (2009) Lung inflammation, injury, and proliferative response after repetitive particulate hexavalent chromium exposure. Environ. Health Perspect. 117 (12): 1896–1902. DOI: 10.1289/ehp.0900715.
61. Fleeger AK, Deng JF (2011) A case study of chromium VI-induced skin ulcerations during a porcelain enamel curing operation. Appl. Occup. Environ. Hyg. 5 (6): 1589–1605. DOI: 10.1080/1047322X.1990.10389658.
62. Hessel EVS, Staal YCM, Piersma AH, den Braver-Sewradj SP, Ezendam J (2021) Occupational exposure to hexavalent chromium. Part I. Hazard assessment of non-cancer health effects. Regul. Toxicol. Pharmacol. 126 (105048): 1–18. DOI: 10.1016/j.yrtph.2021.105048.
63. Shelnutt SR, Goad P, Belsito D V. (2007) Dermatological toxicity of hexavalent chromium. Crit. Rev. Toxicol. 37 (5): 375–387.
64. Wedeen RP, Qian L (1991) Chromium-Induced Kidney Disease. Environ. Health Perspect. 92 : 71–74. DOI: 10.2307/3431139.
65. Shrivastava R, Upreti RK, Seth PK, Chaturvedi UC (2002) Effects of chromium on the immune system. FEMS Immunol. Med. Microbiol. 34 (1): 1–7. DOI: 10.1111/J.1574-695X.2002.TB00596.X.
66. Katz SA (1991) The analytical biochemistry of chromium. Environ. Health Perspect. 92 : 13–16. DOI: 10.1289/ehp.919213.
67. Costa M (1997) Toxicity and carcinogenicity of Cr(VI) in animal models and humans. Crit. Rev. Toxicol. 27 (5): 431–442. DOI: 10.3109/10408449709078442.
68. Chiu A, Shi XL, Lee WKP, Hill R, Wakeman TP, Katz A, Xu B, Dalal NS, Robertson JD, Chen C, Chiu N, Donehower L (2010) Review of chromium (VI) apoptosis, cell-cycle-arrest, and carcinogenesis. J. Environ. Sci. Heal. - Part C Environ. Carcinog. Ecotoxicol. Rev. 28 (3): 188–230. DOI: 10.1080/10590501.2010.504980.
69. Jennette KW (1979) Chromate metabolism in liver microsomes. Biol. Trace Elem. Res. 1 (1): 55–62. DOI: 10.1016/0006-291x(78)90931-2.
70. O’Brien T, Xu J, Patierno SR (2001) Effects of glutathione on chromium-induced DNA crosslinking and DNA polymerase arrest. Mol. Cell. Biochem. 222 (1–2): 173–182. DOI: 10.1023/A:1017918330073.
71. Chen QY, Murphy A, Sun H CM (2019) Molecular and epigenetic mechanisms of Cr(VI)-induced carcinogenesis. Toxicol. Appl. Pharmacol. 377 (114636): 1–22. DOI: 10.1016/j.taap.2019.114636.
72. Aiyar J, De Flora S, Wetterhahn KE (1992) Reduction of chromium(vi) to chromium(v) by rat liver cytosolic and microsomal fractions: Is dt-diaphorase involved? Carcinogenesis 13 (7): 1159–1166. DOI: 10.1093/carcin/13.7.1159.
73. Connett PH, Wetterhahn KE (1983) Metabolism of the carcinogen chromate by cellular constituents. Inorg. Elem. Biochem. 54 : 93–124. DOI: 10.1007/bfb0111319.
74. Shi X, Chiu A, Chen CT, Halliwell B, Castranova V, Vallyathan V (1999) Reduction of chromium (VI) and its relationship to carcinogenesis. J. Toxicol. Environ. Heal. - Part B Crit. Rev. 2 (1): 87–104. DOI: 10.1080/109374099281241.
75. Maret W (2019) Chromium supplementation in human health, metabolic syndrome, and diabetes. Met. Ions Life Sci. 19 : 231–251. DOI: 10.1515/9783110527872-015.
76. Wang Y, Su H, Gu Y, Song X, Zhao J (2017) Carcinogenicity of chromium and chemoprevention: A brief update. Onco. Targets Ther. 10: 4065–4079. DOI: 10.2147/OTT.S139262.
77. Senoner T, Dichtl W (2019) Oxidative stress in cardiovascular diseases: Still a therapeutic target? Nutrients 11 (9): 1–25. DOI: 10.3390/nu11092090.
78. Janero DR, Hreniuk D, Sharif HM (1993) Hydrogen peroxide-induced oxidative stress to the mammalian heart-muscle cell (cardiomyocyte): nonperoxidative purine and pyrimidine nucleotide depletion. J. Cell. Physiol. 155 (3): 494–504. DOI: 10.1002/JCP.1041550308.
79. Holmström KM, Finkel T (2014) Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 15 (6): 411–421. DOI: 10.1038/nrm3801.
80. Li JM, Gall NP, Grieve DJ, Chen M, Shah AM (2002) Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension 40 (4): 477–484. DOI: 10.1161/01.HYP.0000032031.30374.32.
81. Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, Shah AM (2003) Increased myocardial NADPH oxidase activity in human heart failure. J. Am. Coll. Cardiol. 41 (12): 2164–2171. DOI: 10.1016/S0735-1097(03)00471-6.
82. Kuroda J, Sadoshima J (2010) NADPH oxidase and cardiac failure. J. Cardiovasc. Transl. Res. 3 (4): 314–320. DOI: 10.1007/s12265-010-9184-8.
83. Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J (2010) NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc. Natl. Acad. Sci. U. S. A. 107 (35): 15565–15570. DOI: 10.1073/pnas.1002178107.
84. Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J (2010) Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res. 106 (7): 1253–1264. DOI: 10.1161/CIRCRESAHA.109.213116.
85. Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 18 (2): 321–336. DOI: 10.1016/0891-5849(94)00159-H.
86. Kanner J, German JB, Kinsella JE (1987) Initiation of lipid peroxidation in biological systems. C R C Crit. Rev. Food Sci. Nutr. 25 (4): 317–364. DOI: 10.1080/10408398709527457.
87. Halliwell B, Chirico S (1993) Lipid peroxidation: its mechanism, measurement, and significance. Am. J. Clin. Nutr. 57 (5): 715–725. DOI: 10.1093/ajcn/57.5.715S.
88. Upadhyay RK, Panda SK (2010) Influence of chromium salts on increased lipid peroxidation and differential pattern in antioxidant metabolism in Pistia stratiotes L. Brazilian Arch. Biol. Technol. 53 (5): 1137–1144. DOI: 10.1590/S1516-89132010000500008.
89. Katz AM, Freston JW, Messineo FC, Herbette LG (1985) Membrane damage and the pathogenesis of cardiomyopathies. J. Mol. Cell. Cardiol. 17 (SUPPL. 2): 11–20. DOI: 10.1016/0022-2828(85)90004-5.
90. Schwenke DC, Carew TE (1989) Initiation of atherosclerotic lesions in cholesterol-fed rabbits. II. Selective retention of LDL vs. selective increases in LDL permeability in susceptible sites of arteries. Arteriosclerosis 9 (6): 908–918. DOI: 10.1161/01.ATV.9.6.908.
91. Wang X, Son YO, Chang Q, Sun L, Hitron JA, Budhraja A, Zhang Z, Ke Z, Chen F, Luo J, Shi X (2011) NADPH oxidase activation is required in reactive oxygen species generation and cell transformation induced by hexavalent chromium. Toxicol. Sci. 123 (2): 399–410. DOI: 10.1093/toxsci/kfr180.
92. Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, Nishigaki I (2013) The vascular endothelium and human diseases. Int. J. Biol. Sci. 9 (10): 1057–1069. DOI: 10.7150/ijbs.7502.
93. Harrison DG (1997) Cellular and molecular mechanisms of endothelial cell dysfunction. J. Clin. Invest. 100 (9): 2153–2157. DOI: 10.1172/JCI119751.
94. Salvador M, Higgs A (1992) The l-arginine-nitric oxide pathway. N. Engl. J. Med. 397 (27): 2002–2012. DOI: 10.1056/NEJM199312303292706.
95. Forstermann U, Münzel T (2006) Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 113 (13): 1708–1714. DOI: 10.1161/CIRCULATIONAHA.105.602532.
96. Kojda G, Harrison D (1999) Interactions between NO and reactive oxygen species: Pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc. Res. 43 (3): 562–571. DOI: 10.1016/S0008-6363(99)00169-8.
97. Bloodsworth A, Donnell VBO, Freeman B (2000) Nitric oxide regulation of free radical– and enzyme-mediated lipid and lipoprotein oxidation. Arterioscler. Thromb. 20 (7): 1707–1715. DOI: 10.1161/01.atv.20.7.1707.
98. Thomson L, Trujillo M, Telleri R, Radi R (1995) Kinetics of cytochrome c2+ oxidation by peroxynitrite implications for superoxide measurements in nitric oxide-producing biological systems. Arch. Biochem. Biophys. 319 (2): 491–497. DOI: 10.1006/abbi.1995.1321.
99. Touyz RM (2004) Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: What is the clinical significance? Hypertension 44 (3): 248–252. DOI: 10.1161/01.HYP.0000138070.47616.9d.
100. Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA (1990) Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U. S. A. 87 (4): 1620–1624. DOI: 10.1073/pnas.87.4.1620.
101. White CR, Brock TA, Chang LY, Crapo J, Briscoe P, Ku D, Bradley WA, Gianturco SH, Gore J, Freeman BA, Tarpey MM (1994) Superoxide and peroxynitrite in atherosclerosis. Proc. Natl. Acad. Sci. U. S. A. 91 (3): 1044–1048. DOI: 10.1073/pnas.91.3.1044.
102. Radi R, Rodriguez M, Castro L, Telleri R (1994) Inhibition of mitochondrial electron transport by peroxynitrite. Arch. Biochem. Biophys. 308 (1): 89–95. DOI: 10.1006/abbi.1994.1013.
103. Castro L, Rodriguez M, Radi R (1994) Aconitase is readily inactivated by peroxynitrite, but not by its precursor, nitric oxide. J. Biol. Chem. 269 (47): 29409–29415. DOI: 10.1016/s0021-9258(18)43894-x.
104. Sellke FW, Armstrong ML, Harrison DG (1990) Endothelium-dependment vascular relaxation is abnormal in the coronary microcirculation of atherosclerotic primates. Circulation 81 (5): 1586–1593. DOI: 10.1161/01.CIR.81.5.1586.
105. Zeiher AM, Drexler H, Wollschlager H, Just H (1991) Endothelial dysfunction of the coronary microvasculature is associated with impaired coronary blood flow regulation in patients with early atherosclerosis. Circulation 84 (5): 1984–1992. DOI: 10.1161/01.CIR.84.5.1984.
106. Creager MA, Cooke JP, Mendelsohn ME, Gallagher SJ, Coleman SM, Loscalzo J, Dzau VJ (1990) Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J. Clin. Invest. 86 (1): 228–234. DOI: 10.1172/JCI114688.
107. Oishi K, Raynor RL, Charp PA, Kuo JF (1988) Regulation of protein kinase C by lysophospholipids: Potential role in signal transduction. J. Biol. Chem. 263 (14): 6865–6871. DOI: 10.1016/s0021-9258(18)68724-1.
108. Ohara Y, Peterson TE, Zheng B, Kuo JF, Harrison DG (1994) Lysophosphatidylcholine increases vascular superoxide anion production via protein kinase C activation. Arterioscler. Thromb. Vasc. Biol. 14 (6): 1007–1013. DOI: 10.1161/01.ATV.14.6.1007.
109. Pritchard J, Ackerman A, Kalyanaraman B (2000) Chromium (VI) increases endothelial cell expression of ICAM-1 and decreases nitric oxide activity. J. Environ. Pathol. Toxicol. Oncol. 19 (3): 251–260.
110. Porter R, Jáchymová M, Martásek P, Kalyanaraman B, Vásquez-Vivar J (2005) Reductive activation of Cr(VI) by nitric oxide synthase. Chem. Res. Toxicol. 18 (5): 834–843. DOI: 10.1021/TX049778E.
111. Kushwaha BK, Ali HM, Siddiqui MH, Singh VP (2020) Nitric oxide-mediated regulation of sub-cellular chromium distribution, ascorbate-glutathione cycle and glutathione biosynthesis in tomato roots under chromium (VI) toxicity. J. Biotechnol. 318 (2): 68–77. DOI: 10.1016/J.JBIOTEC.2020.05.006.
112. Brito R, Castillo G, González J, Valls N, Rodrigo R (2015) Oxidative stress in hypertension: Mechanisms and therapeutic opportunities. Exp. Clin. Endocrinol. Diabetes 123 (6): 325–335. DOI: 10.1055/s-0035-1548765.
113. Görlach A, Bertram K, Hudecova S, Krizanova O (2015) Calcium and ROS: A mutual interplay. Redox Biol. 6 : 260–271. DOI: 10.1016/j.redox.2015.08.010.
114. Fiolet J, Baartscheer A, Schumacher C, Coronel R, Terwelle H (1984) The change of the free energy of ATP hydrolysis during global ischemia and anoxia in the rat heartIts possible role in the regulation of transsarcolemmal sodium and potassium gradients. J. Mol. Cell. Cardiol. 16 (11): 1023–1036. DOI: 10.1016/S0022-2828(84)80015-2.
115. Vanechteld C, Kirkels J, Eijgelshoven M, Vandermeer P, Ruigrok T (1991) Intracellular sodium during ischemia and calcium-free perfusion: A 23Na NMR study. J. Mol. Cell. Cardiol. 23 (3): 297–307. DOI: 10.1016/0022-2828(91)90066-U.
116. Philipson KD, Ward R (1985) Effects of fatty acids on Na+-Ca2+ exchange and Ca2+ permeability of cardiac sarcolemmal vesicles. J. Biol. Chem. 260 (17): 9666–9671. DOI: 10.1016/s0021-9258(17)39290-6.
117. Dhalla NS, Elimban V, Rupp H (1992) Paradoxical role of lipid metabolism in heart function and dysfunction. Mol. Cell. Biochem. 116 (1–2): 3–9. DOI: 10.1007/BF01270562.
118. Tani M (1990) Mechanisms of Ca2+ overload in reperfused ischemic myocardium. Annu. Rev. Physiol. 52 (1): 543–559. DOI: 10.1146/annurev.physiol.52.1.543.
119. Mukherjee A, Wong TM, Buja LM, Lefkowitz RJ, Willerson JT (1979) Beta adrenergic and muscarinic cholinergic receptors in canine myocardium: Effects of ischemia. J. Clin. Invest. 64 (5): 1423–1428. DOI: 10.1172/JCI109600.
120. Dhalla NS, Temsah RM, Netticadan T (2000) Role of oxidative stress in cardiovascular diseases. J. Hypertens. 18 (6): 655–673. DOI: 10.1097/00004872-200018060-00002.
121. Ishida H, Ichimori K, Hirota Y, Fukahori M, Nakazawa H (1996) Peroxynitrite-induced cardiac myocyte injury. Free Radic. Biol. Med. 20 (3): 343–350. DOI: 10.1016/0891-5849(96)02060-6.
122. Guerini D, Coletto L, Carafoli E (2005) Exporting calcium from cells. Cell Calcium. 38 (3–4): 281–289. DOI: 10.1016/j.ceca.2005.06.032.
123. Xie Y, Xiao F, Luo L, Zhong C (2014) Activation of autophagy protects against ROS-mediated mitochondria-dependent apoptosis in L-02 hepatocytes induced by Cr(VI). Cell. Physiol. Biochem. 33 (3): 705–716. DOI: 10.1159/000358646.
124. Kaplan JH (2003) Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 71: 511–535. DOI: 10.1146/ANNUREV.BIOCHEM.71.102201.141218.
125. Cheung WY (1980) Calmodulin plays a pivotal role in cellular regulation. Science 207 (4426): 19–27. DOI: 10.1126/SCIENCE.6243188.
126. Zhang X, Wang Y, Chen M, Zeng M (2021) Hexavalent chromium-induced apoptosis in Hep3B cells is accompanied by calcium overload, mitochondrial damage, and AIF translocation. Ecotoxicol. Environ. Saf. 208 (111391): 1–10. DOI: 10.1016/j.ecoenv.2020.111391.
127. Liang Q, Zhang Y, Zeng M, Guan L, Xiao Y, Xiao F (2018) The role of IP3R-SOCCs in Cr(vi)-induced cytosolic Ca 2+ overload and apoptosis in L-02 hepatocytes. Toxicol. Res. (Camb). 7 (3): 521–528. DOI: 10.1039/C8TX00029H.
128. Finkel T, Deng CX, Mostoslavsky R (2009) Recent progress in the biology and physiology of sirtuins. Nature 460 (7255): 587–591. DOI: 10.1038/nature08197.
129. Matsushima S, Sadoshima J (2015) The role of sirtuins in cardiac disease. Am. J. Physiol. - Hear. Circ. Physiol. 309 (9): H1375–H1389. DOI: 10.1152/AJPHEART.00053.2015.
130. Xu S, Bai P, Jin ZG (2016) Sirtuins in cardiovascular health and diseases. Trends Endocrinol. Metab. 27 (10): 677–678. DOI: 10.1016/j.tem.2016.07.004.
131. Han B, Li S, Lv Y, Yang D, Li J, Yang Q, Wu P, Lv Z, Zhang Z (2019) Dietary melatonin attenuates chromium-induced lung injury via activating the Sirt1/Pgc-1α/Nrf2 pathway. Food Funct. 10 (9): 5555–5565. DOI: 10.1039/C9FO01152H.
132. Yang Q, Han B, Li S, Wang X, Wu P, Liu Y, Li J, Han B, Deng N, Zhang Z (2022) The link between deacetylation and hepatotoxicity induced by exposure to hexavalent chromium. J. Adv. Res. 35 : 129–140. DOI: 10.1016/j.jare.2021.04.002.
133. Shil K, Pal S (2018) Metabolic adaptability in hexavalent chromium-treated renal tissue: an in vivo study. Clin. Kidney J. 11 (2): 222–229. DOI: 10.1093/ckj/sfx069.
134. Tanno M, Kuno A, Horio Y, Miura T (2012) Emerging beneficial roles of sirtuins in heart failure. Basic Res. Cardiol. 107 (4): 1–14. DOI: 10.1007/s00395-012-0273-5.
135. Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, Garcia JA (2009) Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science (80-. ). 324 (5932): 1289–1293. DOI: 10.1126/SCIENCE.1169956.
136. Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M, Nakajima T, Fukamizu A (2004) Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc. Natl. Acad. Sci. U. S. A. 101 (27): 10042–10047. DOI: 10.1073/PNAS.0400593101.
137. Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP (2009) Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J. Clin. Invest. 119 (9): 2758–2771. DOI: 10.1172/JCI39162.
138. Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J (2007) Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ. Res. 100 (10): 1512–1521. DOI: 10.1161/01.RES.0000267723.65696.4A.
139. Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J (2010) Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation 122 (21): 2170–2182. DOI: 10.1161/CIRCULATIONAHA.110.958033.
140. Ahmad MK, Syma S, Mahmood R (2011) Cr(VI) induces lipid peroxidation, protein oxidation and alters the activities of antioxidant enzymes in human erythrocytes. Biol. Trace Elem. Res. 144 (1–3): 426–435. DOI: 10.1007/s12011-011-9119-5.
141. Kalahasthi RB, Rao RHR, Murthy RBK, Kumar MK (2006) Effect of chromium(VI) on the status of plasma lipid peroxidation and erythrocyte antioxidant enzymes in chromium plating workers. Chem. Biol. Interact. 164 (3): 192–199. DOI: 10.1016/j.cbi.2006.09.012.
142. Wang YP, Zhou LS, Zhao YZ, Wang SW, Chen LL, Liu LX, Ling ZQ, Hu FJ, Sun YP, Zhang JY, Yang C, Yang Y, Xiong Y, Guan KL, Ye D (2014) Regulation of G6PD acetylation by SIRT2 and KAT9 modulates NADPH homeostasis and cell survival during oxidative stress. EMBO J. 33 (12): 1304–1320. DOI: 10.1002/EMBJ.201387224.
143. Cupo DY, Wetterhahn KE (1985) Modification of chromium(VI)-induced DNA damage by glutathione and cytochromes P-450 in chicken embryo hepatocytes. Proc. Natl. Acad. Sci. U. S. A. 82 (20): 6755–6759. DOI: 10.1073/pnas.82.20.6755.
144. Finley LWS, Haas W, Desquiret-Dumas V, Wallace DC, Procaccio V, Gygi SP, Haigis MC (2011) Succinate dehydrogenase is a direct target of sirtuin 3 deacetylase activity. PLoS One 6 (8): 1–6. DOI: 10.1371/journal.pone.0023295.
145. Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA (2010) Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143 (5): 802–812. DOI: 10.1016/j.cell.2010.10.002.
146. Lombard DB, Alt FW, Cheng H-L, Bunkenborg J, Streeper RS, Mostoslavsky R et al. (2007) Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol. Cell. Biol. 27 (24): 8807–8814. DOI: 10.1128/MCB.01636-07.
147. Giralt A, Villarroya F (2012) SIRT3, a pivotal actor in mitochondrial functions: metabolism, cell death and aging. Biochem. J. 444 (1): 1–10. DOI: 10.1042/BJ20120030.
148. Finley LWS, Haigis MC (2012) Metabolic regulation by SIRT3: implications for tumorigenesis. Trends Mol. Med. 18 (9): 516–523. DOI: 10.1016/j.molmed.2012.05.004.
149. Ahn B-H, Kim H-S, Song S, Lee IH, Liu J, Vassilopoulos A, Deng C-X, Finkel T (2008) A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl. Acad. Sci. U.S.A. 105 (38): 14447–14452. DOI: 10.1073/pnas.0803790105.
150. Clementino M, Kim D, Zhang Z (2019) Constitutive activation of NAD-dependent sirtuin 3 plays an important role in tumorigenesis of chromium(VI)-transformed cells. Toxicol. Sci. 169 (1): 224–234. DOI: 10.1093/toxsci/kfz032.
151. Chauhan AS, Zhuang L, Gan B (2020) Spatial control of AMPK signaling at subcellular compartments. Crit. Rev. Biochem. Mol. Biol. 55 (1): 17–32. DOI: 10.1080/10409238.2020.1727840.
152. Cantó C, Auwerx J (2009) PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 20 (2): 98–105. DOI: 10.1097/MOL.0B013E328328D0A4.
153. Ojuka EO, Nolte LA, Holloszy JO (2000) Increased expression of GLUT-4 and hexokinase in rat epitrochlearis muscles exposed to AICAR in vitro. J. Appl. Physiol. 88 (3): 1072–1075. DOI: 10.1152/JAPPL.2000.88.3.1072.
154. Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO (2000) Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J. Appl. Physiol. 88 (6): 2219–2226. DOI: 10.1152/JAPPL.2000.88.6.2219.
155. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108 (8): 1167–1174. DOI: 10.1172/JCI13505.
156. Ma Y, Li S, Ye S, Tang S, Hu D, Wei L, Xiao F (2021) Hexavalent chromium inhibits the formation of neutrophil extracellular traps and promotes the apoptosis of neutrophils via AMPK signaling pathway. Ecotoxicol. Environ. Saf. 223 (112614): 1–12. DOI: 10.1016/j.ecoenv.2021.112614.
157. Wang X-X, Wang X-L, Tong M, Gan L, Chen H, Wu S, Chen J-X, Li R-L, Wu Y, Zhang H, Zhu Y, Li Y, He J, Wang M, Jiang W (2016) SIRT6 protects cardiomyocytes against ischemia/reperfusion injury by augmenting FoxO3α-dependent antioxidant defense mechanisms. Basic Res. Cardiol. 111 (2): 1–19. DOI: 10.1007/s00395-016-0531-z.
158. Chiacchiera A F, Simone C (2010) The AMPK-FoxO3A axis as a target for cancer treatment. Cell Cycle 9 (6): 1091–1096. DOI: 10.4161/CC.9.6.11035.
159. Mori S, Nada S, Kimura H, Tajima S, Takahashi Y, Kitamura A, Oneyama C, Okada M (2014) The mTOR pathway controls cell proliferation by regulating the FoxO3a transcription factor via SGK1 kinase. PLoS One 9 (2): 1–12. DOI: 10.1371/journal.pone.0088891.
160. Cantó C, Auwerx J (2009) PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 20 (2): 98–105. DOI: 10.1097/MOL.0b013e328328d0a4.
161. Liang H, Ward WF (2006) PGC-1α: a key regulator of energy metabolism. Adv. Physiol. Educ. 30 (4): 145–151. DOI: 10.1152/advan.00052.2006.
162. Onishi Y, Ueha T, Kawamoto T, Hara H, Toda M, Harada R, Minoda M, Kurosaka M, Akisue T (2015) Regulation of mitochondrial proliferation by PGC-1α induces cellular apoptosis in musculoskeletal malignancies. Sci. Rep. 4 (3916): 1–8. DOI: 10.1038/srep03916.
163. Jäer S, Handschin C, St-Pierre J, Spiegelman BM (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. U. S. A. 104 (29): 12017–12022. DOI: 10.1073/PNAS.0705070104.
164. Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P (2005) Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434 (7029): 113–118. DOI: 10.1038/NATURE03354.
165. Nemoto S, Fergusson MM, Finkel T (2005) SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J. Biol. Chem. 280 (16): 16456–16460. DOI: 10.1074/jbc.M501485200.
166. Teyssier C, Ma H, Emter R, Kralli A, Stallcup MR (2005) Activation of nuclear receptor coactivator PGC-1α by arginine methylation. Genes Dev. 19 (12): 1466–1472. DOI: 10.1101/GAD.1295005.
167. Furukawa A, Tada-Oikawa S, Kawanishi S, Oikawa S (2007) H2O2 accelerates cellular senescence by accumulation of acetylated p53 via decrease in the function of SIRT1 by NAD+ depletion. Cell. Physiol. Biochem. 20 (1–4): 045–054. DOI: 10.1159/000104152.
168. Wang Y, Meng A, Zhou D (2004) Inhibition of phosphatidylinostol 3-kinase uncouples H2O2-induced senescent phenotype and cell cycle arrest in normal human diploid fibroblasts. Exp. Cell Res. 298 (1): 188–196. DOI: 10.1016/j.yexcr.2004.04.012.
169. Sablina AA, Budanov A V., Ilyinskaya G V., Agapova LS, Kravchenko JE, Chumakov PM (2005) The antioxidant function of the p53 tumor suppressor. Nat. Med. 11 (12): 1306–1313. DOI: 10.1038/nm1320.
170. Luo J, Li M, Tang Y, Laszkowska M, Roeder RG, Gu W (2004) Acetylation of p53 augments its site-specific DNA binding both in vitro and in vivo. Proc. Natl. Acad. Sci. 101 (8): 2259–2264. DOI: 10.1073/pnas.0308762101.
171. Davidoff AM, Herndon JE, Glover NS, Kerns BJ, Pence JC, Lglehart JD MJ (1991) Relation between p53 overexpression and established prognostic factors in breast cancer. Surgery. 110 (2): 259–264.
172. Ramadan MA, Shawkey AE, Rabeh MA, Abdellatif AO (2019) Expression of P53, BAX, and BCL-2 in human malignant melanoma and squamous cell carcinoma cells after tea tree oil treatment in vitro. Cytotechnology 71 (1): 461–473. DOI: 10.1007/S10616-018-0287-4.
173. Gahl RF, Dwivedi P, Tjandra N (2016) Bcl-2 proteins bid and bax form a network to permeabilize the mitochondria at the onset of apoptosis. Cell Death Dis. 7 (10): e2424–e2424. DOI: 10.1038/cddis.2016.320.
174. Li H, Shi J, Gao H, Yang X, Fu Y, Peng Y, Xia Y, Zhou D (2021) Hexavalent chromium causes apoptosis and autophagy by inducing mitochondrial dysfunction and oxidative stress in broiler cardiomyocytes. Biol. Trace Elem. Res. 200 (6): 1–10. DOI: 10.1007/S12011-021-02877-X/FIGURES/6.
175. Schuler M, Bossy-Wetzel E, Goldstein JC, Fitzgerald P, Green DR (2000) p53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. J. Biol. Chem. 275 (10): 7337–7342. DOI: 10.1074/JBC.275.10.7337.
176. Narula J, Pandey P, Arbustini E, Haider N, Narula N, Kolodgie FD, Dal Bello B, Semigran MJ, Bielsa-Masdeu A, Dec GW, Israels S, Ballester M, Virmani R, Saxena S, Kharbanda S (1999) Apoptosis in heart failure: Release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc. Natl. Acad. Sci. U. S. A. 96 (14): 8144–8149. DOI: 10.1073/PNAS.96.14.8144.
177. McIlwain DR, Berger T, Mak TW (2013) Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 5 (4): 1–28. DOI: 10.1101/CSHPERSPECT.A008656.
178. Moorjani N, Ahmad M, Catarino P, Brittin R, Trabzuni D, Al-Mohanna F, Narula N, Narula J, Westaby S (2006) Activation of apoptotic caspase cascade during the ansition to pressure overload-induced heart failure. J. Am. Coll. Cardiol. 48 (7): 1451–1458. DOI: 10.1016/j.jacc.2006.05.065.
179. Yang B, Ye D, Wang Y (2013) Caspase-3 as a therapeutic target for heart failure. Expert Opin. Ther. Targets 17 (3): 255–263. DOI: 10.1517/14728222.2013.745513.
180. Li J, Zheng X, Ma X, Xu X, Du Y, Lv Q, Li X, Wu Y, Sun H, Yu L, Zhang Z (2019) Melatonin protects against chromium(VI)-induced cardiac injury via activating the AMPK/Nrf2 pathway. J. Inorg. Biochem. 197 (110698): 1–9. DOI: 10.1016/j.jinorgbio.2019.110698.
181. Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, Braun T, Bober E (2008) Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ. Res. 102 (6): 703–710. DOI: 10.1161/CIRCRESAHA.107.164558.
182. Sivakumar KK, Stanley JA, Behlen JC, Wuri L, Dutta S, Wu J, Arosh JA, Banu SK (2022) Inhibition of Sirtuin-1 hyperacetylates p53 and abrogates Sirtuin-1-p53 interaction in Cr(VI)-induced apoptosis in the ovary. Reprod. Toxicol. 109 : 121–134. DOI: 10.1016/J.REPROTOX.2022.03.007.
183. Wang S, Leonard SS, Ye J, Gao N, Wang L, Shi X (2004) Role of reactive oxygen species and Cr(VI) in Ras-mediated signal transduction. Mol. Cell. Biochem. 255 (1–2): 119–127. DOI: 10.1023/B:MCBI.0000007268.42733.53.
184. Ganapathy S, Li P, Lafontant J, Xiong R, Yu T, Zhang G, Chen C (2017) Chromium IV exposure, via Src/Ras signaling, promotes cell transformation. Mol. Carcinog. 56 (7): 1808–1815. DOI: 10.1002/MC.22639.
185. Castellano E, Downward J (2011) RAS interaction with PI3K: More than just another effector pathway. Genes Cancer 2 (3): 261–274. DOI: 10.1177/1947601911408079.
186. Duronio V (2008) The life of a cell: apoptosis regulation by the PI3K/PKB pathway. Biochem. J. 415 (3): 333–344. DOI: 10.1042/BJ20081056.
187. Cantley LC (2002) The phosphoinositide 3-Kinase pathway. Science 296 (5573): 1655–1657. DOI: 10.1126/science.296.5573.1655.
188. Vivanco I, Sawyers CL (2002) The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat. Rev. Cancer 2 (7): 489–501. DOI: 10.1038/NRC839.
189. Cox AD, Der CJ (2003) The dark side of Ras: regulation of apoptosis. Oncogene 22 (56): 8999–9006. DOI: 10.1038/SJ.ONC.1207111.
190. Sundaresan NR, Vasudevan P, Zhong L, Kim G, Samant S, Parekh V, Pillai VB, Ravindra P V., Gupta M, Jeevanandam V, Cunningham JM, Deng C-X, Lombard DB, Mostoslavsky R, Gupta MP (2012) The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat. Med. 18 (11): 1643–1650. DOI: 10.1038/nm.2961.
191. Son YO, Pratheeshkumar P, Wang L, Wang X, Fan J, Kim DH, Lee JY, Zhang Z, Lee JC, Shi X (2013) Reactive oxygen species mediate Cr(VI)-induced carcinogenesis through PI3K/AKT-dependent activation of GSK-3β/β-catenin signaling. Toxicol. Appl. Pharmacol. 271 (2): 239–248. DOI: 10.1016/J.TAAP.2013.04.036.
192. Zhang Y, Zhang Y, Xiao Y, Zhong C, Xiao F (2019) Expression of clusterin suppresses Cr(VI)-induced premature senescence through activation of PI3K/AKT pathway. Ecotoxicol. Environ. Saf. 183 (109465): 1–10. DOI: 10.1016/J.ECOENV.2019.109465.
193. Zhang Y, Xiao F, Liu X, Liu K, Zhou X, Zhong C (2017) Cr(VI) induces cytotoxicity in vitro through activation of ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction via the PI3K/Akt signaling pathway. Toxicol. Vitr. 41 : 232–244. DOI: 10.1016/j.tiv.2017.03.003.
194. Fu S-C, Liu J-M, Lee K-I, Tang F-C, Fang K-M, Yang C-Y, Su C-C, Chen H-H, Hsu R-J, Chen Y-W (2020) Cr(VI) induces ROS-mediated mitochondrial-dependent apoptosis in neuronal cells via the activation of Akt/ERK/AMPK signaling pathway. Toxicol. Vitr. 65 (104795): 104795. DOI: 10.1016/j.tiv.2020.104795.
195. Liang N, Li S, Liang Y, Ma Y, Tang S, Ye S, Xiao F (2021) Clusterin inhibits Cr(VI)-induced apoptosis via enhancing mitochondrial biogenesis through AKT-associated STAT3 activation in L02 hepatocytes. Ecotoxicol. Environ. Saf. 221 (112447): 1–11. DOI: 10.1016/j.ecoenv.2021.112447.
196. Bowman T, Garcia R, Turkson J, Jove R (2000) STATs in oncogenesis. Oncogene 19 (21): 2474–2488. DOI: 10.1038/sj.onc.1203527.
197. Bromberg J, Darnell JE (2000) The role of STATs in transcriptional control and their impact on cellular function. Oncogene 19 (21): 2468–2473. DOI: 10.1038/sj.onc.1203476.
198. Elschami M, Scherr M, Philippens B, Gerardy-Schahn R (2013) Reduction of STAT3 expression induces mitochondrial dysfunction and autophagy in cardiac HL-1 cells. Eur. J. Cell Biol. 92 (1): 21–29. DOI: 10.1016/j.ejcb.2012.09.002.
199. Liang Q, Xiao Y, Liu K, Zhong C, Zeng M, Xiao F (2018) Cr(VI)-induced autophagy protects l-02 hepatocytes from apoptosis through the ROS-AKT-mTOR pathway. Cell. Physiol. Biochem. 51 (4): 1863–1878. DOI: 10.1159/000495713.
200. Chen A, Xiong L-J, Tong Y, Mao M (2013) Neuroprotective effect of brain-derived neurotrophic factor mediated by autophagy through the PI3K/Akt/mTOR pathway. Mol. Med. Rep. 8 (4): 1011–1016. DOI: 10.3892/mmr.2013.1628.
201. Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 160 (1): 1–40. DOI: 10.1016/j.cbi.2005.12.009.
202. Zhao L, Song Y, Pu J, Guo J, Wang Y, Chen Z, Chen T, Gu Y, Jia G (2014) Effects of repeated Cr(VI) intratracheal instillation on club (Clara) cells and activation of nuclear factor-kappa B pathway via oxidative stress. Toxicol. Lett. 231 (1): 72–81. DOI: 10.1016/j.toxlet.2014.09.011.
203. Wang L, Qiu J-G, He J, Liu W-J, Ge X, Zhou F-M, Huang Y-X, Jiang B-H, Liu L-Z (2019) Suppression of miR-143 contributes to overexpression of IL-6, HIF-1α and NF-κB p65 in Cr(VI)-induced human exposure and tumor growth. Toxicol. Appl. Pharmacol. 378 (114603): 1–7. DOI: 10.1016/j.taap.2019.114603.
204. Zhang Y, Yang G, Huang S, Yang X, Yuan F, Song Y, Liu S, Yu X (2022) Regulation of Cr(VI)-induced premature senescence in l02 hepatocytes by ROS-Ca2+-NF-κB signaling. Oxid. Med. Cell. Longev. 2022 (7295224): 1–16. DOI: 10.1155/2022/7295224.
205. Eferl R, Wagner EF (2003) AP-1: A double-edged sword in tumorigenesis. Nat. Rev. Cancer 3 (11): 859–868. DOI: 10.1038/nrc1209.
206. Chen F, Ding M, Lu Y, Leonard SS, Vallyathan V, Castranova V, Shi X (2000) Participation of MAP kinase p38 and IkappaB kinase in chromium (VI)-induced NF-kappaB and AP-1 activation. J. Environ. Pathol. Toxicol. Oncol. 19 (3): 231–238.
207. Fernández A, Ordóñez R, Reiter RJ, González‐Gallego J, Mauriz JL (2015) Melatonin and endoplasmic reticulum stress: relation to autophagy and apoptosis. J. Pineal Res. 59 (3): 292–307. DOI: 10.1111/jpi.12264.
208. Minamino T, Kitakaze M (2010) ER stress in cardiovascular disease. J. Mol. Cell. Cardiol. 48 (6): 1105–1110. DOI: 10.1016/J.YJMCC.2009.10.026.
209. Ge H, Li Z, Jiang L, Li Q, Geng C, Yao X, Shi X, Liu Y, Cao J (2019) Cr (VI) induces crosstalk between apoptosis and autophagy through endoplasmic reticulum stress in A549 cells. Chem. Biol. Interact. 298 : 35–42. DOI: 10.1016/j.cbi.2018.10.024.
210. Liu MQ, Chen Z, Chen LX (2016) Endoplasmic reticulum stress: a novel mechanism and therapeutic target for cardiovascular diseases. Acta Pharmacol. Sin. 37 (4): 425–443. DOI: 10.1038/APS.2015.145.
211. Liang Q, Zhang Y, Huang M, Xiao Y, Xiao F (2019) Role of mitochondrial damage in Cr(VI)‑induced endoplasmic reticulum stress in L‑02 hepatocytes. Mol. Med. Rep. 19 (2): 1256–1265. DOI: 10.3892/MMR.2018.9704.
212. Liu YZ, Wang YX, Jiang CL (2017) Inflammation: The common pathway of stress-related diseases. Front. Hum. Neurosci. 11 (316): 1–11. DOI: 10.3389/FNHUM.2017.00316/BIBTEX.
213. Alfaddagh A, Martin SS, Leucker TM, Michos ED, Blaha MJ, Lowenstein CJ, Jones SR, Toth PP (2020) Inflammation and cardiovascular disease: From mechanisms to therapeutics. Am. J. Prev. Cardiol. 4 (100130): 1–19. DOI: 10.1016/j.ajpc.2020.100130.
214. Shrivastava R, Upreti RK, Seth PK, Chaturvedi UC (2002) Effects of chromium on the immune system. FEMS Immunol. Med. Microbiol. 34 (1): 1–7. DOI: 10.1016/S0928-8244(02)00345-0.
215. Miller AH, Maletic V, Raison CL (2009) Inflammation and Its Discontents: The role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 65 (9): 732–741. DOI: 10.1016/j.biopsych.2008.11.029.
216. Zhang JM, An J (2007) Cytokines, inflammation and pain. Int. Anesthesiol. Clin. 45 (2): 27–37. DOI: 10.1097/AIA.0B013E318034194E.
217. Lee SH, Kwon JY, Kim SY, Jung KA, Cho M La (2017) Interferon-gamma regulates inflammatory cell death by targeting necroptosis in experimental autoimmune arthritis. Sci. Rep. 7 (1): 1–9. DOI: 10.1038/s41598-017-09767-0.
218. Wang Y, Wang L, Wang X, Cheng G, Xing Y, Zhang M, Zhang P, Liu J (2022) Inflammatory injury and mitophagy in the cock heart induced by the oral administration of hexavalent chromium. Biol. Trace Elem. Res. 200 (3): 1312–1320. DOI: 10.1007/s12011-021-02715-0.
219. Hu G, Wang T, Liu J, Chen Z, Zhong L, Yu S, Zhao Z, Zhai M, Jia G (2017) Serum protein expression profiling and bioinformatics analysis in workers occupationally exposed to chromium (VI). Toxicol. Lett. 277: 76–83. DOI: 10.1016/J.TOXLET.2017.05.026.
220. Hu G, Long C, Hu L, Zhang Y, Hong S, Zhang Q, Zheng P, Su Z, Xu J, Wang L, Gao X, Zhu X, Yuan F, Wang T, Yu S, Jia G (2022) Blood chromium exposure, immune inflammation and genetic damage: Exploring associations and mediation effects in chromate exposed population. J. Hazard. Mater. 425 (127769): 1–9. DOI: 10.1016/j.jhazmat.2021.127769.
221. Dai L, Xu W, Li H, Frank JA, He C, Zhang Z, Chen G (2017) Effects of hexavalent chromium on mouse splenic T lymphocytes. Toxicol. Vitr. 45 (1): 166–171. DOI: 10.1016/j.tiv.2017.09.006.
222. Hadrup S, Donia M, Thor Straten P (2012) Effector CD4 and CD8 T cells and their role in the tumor microenvironment. Cancer Microenviron. 6 (2): 123–133. DOI: 10.1007/S12307-012-0127-6.
223. Lee HL, Jang JW, Lee SW, Yoo SH, Kwon JH, Nam SW, Bae SH, Choi JY, Han NI, Yoon SK (2019) Inflammatory cytokines and change of Th1/Th2 balance as prognostic indicators for hepatocellular carcinoma in patients treated with transarterial chemoembolization. Sci. Rep. 9 (1): 1–8. DOI: 10.1038/s41598-019-40078-8.
224. Burastero SE, Paolucci C, Breda D, Ponti J, Munaro B, Sabbioni E (2006) Chromium (VI)-induced immunotoxicity and intracellular accumulation in human primary dendritic cells. Int. J. Immunopathol. Pharmacol. 19 (3): 581–591. DOI: 10.1177/039463200601900314.
225. Eming SA, Krieg T, Davidson JM (2007) Inflammation in wound repair: Molecular and cellular mechanisms. J. Invest. Dermatol. 127 (3): 514–525. DOI: 10.1038/sj.jid.5700701.
226. Freeman CM, Curtis JL, Chensue SW (2007) CC chemokine receptor 5 and CXC chemokine receptor 6 expression by lung CD8+ cells correlates with chronic obstructive pulmonary disease severity. Am. J. Pathol. 171 (3): 767–776. DOI: 10.2353/ajpath.2007.061177.
227. Glaser U, Hochrainer D, Klppel H, Kuhnen H (1985) Low level chromium (VI) inhalation effects on alveolar macrophages and immune functions in Wistar rats. Arch. Toxicol. 57 (4): 250–256. DOI: 10.1007/BF00324787.
228. Tau G, Rothman P (1999) Biologic functions of the IFN‐γ receptors. Allergy 54 (12): 1233–1251. DOI: 10.1034/J.1398-9995.1999.00099.X.
229. Katiyar S, Awasthi SK, Srivastava JK (2009) Effect of chromium on the level of IL-12 and IFN-gamma in occupationally exposed workers. Sci. Total Environ. 407 (6): 1868–1874. DOI: 10.1016/j.scitotenv.2008.11.057.
230. Tabas I, Lichtman AH (2017) Monocyte-macrophages and T cells in atherosclerosis. Immunity 47 (4): 621–634. DOI: 10.1016/j.immuni.2017.09.008.
231. Teixeira LK, Fonseca BPF, Barboza BA, Viola JPB (2005) The role of interferon-gamma on immune and allergic responses. Mem. Inst. Oswaldo Cruz. 100 (suppl 1): 137–144. DOI: 10.1590/S0074-02762005000900024.
232. Ovchinnikova O, Robertson A-KL, Wågsäter D, Folco EJ, Hyry M, Myllyharju J, Eriksson P, Libby P, Hansson GK (2009) T-cell activation leads to reduced collagen maturation in atherosclerotic plaques of Apoe−/− mice. Am. J. Pathol. 174 (2): 693–700. DOI: 10.2353/ajpath.2009.080561.
233. Bennett MR, Sinha S, Owens GK (2016) Vascular smooth muscle cells in atherosclerosis. Circ. Res. 118 (4): 692–702. DOI: 10.1161/CIRCRESAHA.115.306361.
234. Michael Garavito R, Malkowski MG, DeWitt DL (2002) The structures of prostaglandin endoperoxide H synthases-1 and -2. Prostaglandins Other Lipid Mediat. 68–69 : 129–152. DOI: 10.1016/S0090-6980(02)00026-6.
235. Patrono C (2016) Cardiovascular effects of cyclooxygenase-2 inhibitors: a mechanistic and clinical perspective. Br. J. Clin. Pharmacol. 82 (4): 957–964. DOI: 10.1111/bcp.13048.
236. Smith WL, Langenbach R (2001) Why there are two cyclooxygenase isozymes. J. Clin. Invest. 107 (12): 1491–1495. DOI: 10.1172/JCI13271.
237. Grosser T (2005) Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J. Clin. Invest. 116 (1): 4–15. DOI: 10.1172/JCI27291.
238. Chen P, Geng N, Zhou D, Zhu Y, Xu Y, Liu K, Liu Y, Liu J (2019) The regulatory role of COX-2 in the interaction between Cr(VI)-induced endoplasmic reticulum stress and autophagy in DF-1 cells. Ecotoxicol. Environ. Saf. 170: 112–119. DOI: 10.1016/J.ECOENV.2018.11.120.
239. Klionsky DJ, Abdel-Aziz AK, Abdelfatah S, Abdellatif M, Abdoli A, Abel S et al. (2021) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 17 (1): 1–382.
240. Yorimitsu T, Nair U, Yang Z, Klionsky DJ (2006) Endoplasmic reticulum stress triggers autophagy. J. Biol. Chem. 281 (40): 30299–30304. DOI: 10.1074/JBC.M607007200.
241. Murrow L, Debnath J (2013) Autophagy as a stress-response and quality-control mechanism: Implications for cell injury and human disease. Annu. Rev. Pathol. 8 : 105–137. DOI: 10.1146/ANNUREV-PATHOL-020712-163918.
242. Luo C, He M, Bohlin L (2005) Is COX-2 a perpetrator or a protector? Selective COX-2 inhibitors remain controversial. Acta Pharmacol. Sin. 26 (8): 926–933. DOI: 10.1111/j.1745-7254.2005.00150.x.
243. Angeli JK, Cruz Pereira CA, de Oliveira Faria T, Stefanon I, Padilha AS, Vassallo DV (2013) Cadmium exposure induces vascular injury due to endothelial oxidative stress: the role of local angiotensin II and COX-2. Free Radic. Biol. Med. 65 : 838–848. DOI: 10.1016/j.freeradbiomed.2013.08.167.
244. Liu T, Zhang L, Joo D, Sun S-C (2017) NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2 (17023): 1–9. DOI: 10.1038/sigtrans.2017.23.
245. Anandasadagopan SK, Sundaramoorthy C, Pandurangan AK, Nagarajan V, Srinivasan K, Ganapasam S (2017) S-Allyl cysteine alleviates inflammation by modulating the expression of NF-ΰB during chromium (VI)-induced hepatotoxicity in rats. Hum. Exp. Toxicol. 36 (11): 1186–1200. DOI: 10.1177/0960327116680275.
246. Gordon JW, Shaw JA, Kirshenbaum LA (2011) Multiple facets of NF-κB in the heart. Circ. Res. 108 (9): 1122–1132. DOI: 10.1161/CIRCRESAHA.110.226928.
247. Zhao D, Yu Y, Shen Y, Liu Q, Zhao Z, Sharma R, Reiter RJ (2019) Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. (Lausanne). 10 (249): 1–16. DOI: 10.3389/FENDO.2019.00249/BIBTEX.
248. Reiter RJ, Tan DX, Zhou Z, Cruz MHC, Fuentes-Broto L, Galano A (2015) Phytomelatonin: Assisting plants to survive and thrive. Molecules 20 (4): 7396–7437. DOI: 10.3390/molecules20047396.
249. Reiter RJ, Mayo JC, Tan D-X, Sainz RM, Alatorre-Jimenez M, Qin L (2016) Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 61 (3): 253–278. DOI: 10.1111/jpi.12360.
250. Sharman EH, Bondy SC (2016) Melatonin: A safe nutraceutical and clinical agent. Nutraceuticals: efficacy, safety and toxicity. Elsevier; 2016. 501–509 p. DOI: 10.1016/B978-0-12-802147-7.00036-X.
251. Romero A, Ramos E, De Los Ríos C, Egea J, Del Pino J, Reiter RJ (2014) A review of metal-catalyzed molecular damage: Protection by melatonin. J. Pineal Res. 56 (4): 343–370. DOI: 10.1111/jpi.12132.
252. Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan D-X, Reiter RJ (2014) Extrapineal melatonin: sources, regulation, and potential functions. Cell. Mol. Life Sci. 71 (16): 2997–3025. DOI: 10.1007/s00018-014-1579-2.
253. Sun H, Gusdon AM, Qu S (2016) Effects of melatonin on cardiovascular diseases. Curr. Opin. Lipidol. 27 (4): 408–413. DOI: 10.1097/MOL.0000000000000314.
254. Pandi-Perumal SR, BaHammam AS, Ojike NI, Akinseye OA, Kendzerska T, Buttoo K, Dhandapany PS, Brown GM, Cardinali DP (2016) Melatonin and human cardiovascular disease: J. Cardiovasc. Pharmacol. Ther. 22 (2): 122–132. DOI: 10.1177/1074248416660622.
255. Sewerynek E (2002) Melatonin and the cardiovascular system. Neuroendocrinol. Lett. 4 (suppl 1): 79–83.
256. Liu J, Clough SJ, Hutchinson AJ, Adamah-Biassi EB, Popovska-Gorevski M, Dubocovich ML (2016) MT 1 and MT 2 melatonin receptors: A therapeutic perspective. Annu. Rev. Pharmacol. Toxicol. 56 (1): 361–383. DOI: 10.1146/annurev-pharmtox-010814-124742.
257. Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR (2011) Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 93 (3): 350–384. DOI: 10.1016/j.pneurobio.2010.12.004.
258. Grossini E, Molinari C, Uberti F, Mary DASG, Vacca G, Caimmi PP (2011) Intracoronary melatonin increases coronary blood flow and cardiac function through β-adrenoreceptors, MT1/MT2 receptors, and nitric oxide in anesthetized pigs. J. Pineal Res. 51 (2): 246–257. DOI: 10.1111/J.1600-079X.2011.00886.X.
259. Han D, Wang Y, Chen J, Zhang J, Yu P, Zhang R, Li S, Tao B, Wang Y, Qiu Y, Xu M, Gao E, Cao F (2019) Activation of melatonin receptor 2 but not melatonin receptor 1 mediates melatonin-conferred cardioprotection against myocardial ischemia/reperfusion injury. J. Pineal Res. 67 (1): e12571. DOI: 10.1111/JPI.12571.
260. Ekmekcioglu C, Thalhammer T, Humpeler S, Mehrabi MR, Glogar HD, Hölzenbein T, Markovic O, Leibetseder VJ, Strauss-Blasche G, Marktl W (2003) The melatonin receptor subtype MT2 is present in the human cardiovascular system. J. Pineal Res. 35 (1): 40–44. DOI: 10.1034/J.1600-079X.2003.00051.X.
261. Scheer FAJL, Van Montfrans GA, Van Someren EJW, Mairuhu G, Buijs RM (2004) Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension 43 (2): 192–197. DOI: 10.1161/01.HYP.0000113293.15186.3B.
262. Grossman E, Laudon M, Yalcin R, Zengil H, Peleg E, Sharabi Y, Kamari Y, Shen-Orr Z, Zisapel N (2006) Melatonin reduces night blood pressure in patients with nocturnal hypertension. Am. J. Med. 119 (10): 898–902. DOI: 10.1016/J.AMJMED.2006.02.002.
263. Katsi V, Karagiorgi I, Makris T, Papavasileiou M, Androulakis AE, Tsioufis C, Tousoulis D, Stefanadis C, Kallikazaros IE (2012) The role of melatonin in hypertension: A brief review. Cardiovasc. Endocrinol. 1 (1): 13–18. DOI: 10.1097/XCE.0B013E3283565783.
264. Simko F, Pechanova O (2009) Potential roles of melatonin and chronotherapy among the new trends in hypertension treatment. J. Pineal Res. 47 (2): 127–133. DOI: 10.1111/J.1600-079X.2009.00697.X.
265. Paulis L, Šimko F (2007) Blood pressure modulation and cardiovascular protection by melatonin: potential mechanisms behind. Physiol. Res. 56 (6): 671–684. DOI: 10.33549/PHYSIOLRES.931236.
266. Ray CA (2003) Melatonin attenuates the sympathetic nerve responses to orthostatic stress in humans. J. Physiol. 551 (Pt 3): 1043–1048. DOI: 10.1113/JPHYSIOL.2003.043182.
267. Nishiyama K, Yasue H, Moriyama Y, Tsunoda R, Ogawa H, Yoshimura M, Kugiyama K (2001) Acute effects of melatonin administration on cardiovascular autonomic regulation in healthy men. Am. Heart J. 141 (5): 1–5. DOI: 10.1067/MHJ.2001.114368.
268. Chan TY, Tang PL (1996) Characterization of the antioxidant effects of melatonin and related indoleamines in vitro. J. Pineal Res. 20 (4): 187–191. DOI: 10.1111/j.1600-079X.1996.tb00257.x.
269. Mayo JC, Tan DX, Sainz RM, Lopez-Burillo S, Reiter RJ (2003) Oxidative damage to catalase induced by peroxyl radicals: Functional protection by melatonin and other antioxidants. Free Radic. Res. 37 (5): 543–553. DOI: 10.1080/1071576031000083206.
270. Marshall KA, Reiter RJ, Poeggeler B, Aruoma OI, Halliwell B (1996) Evaluation of the antioxidant activity of melatonin in vitro. Free Radic. Biol. Med. 21 (3): 307–315. DOI: 10.1016/0891-5849(96)00046-9.
271. Stasica P, Paneth P, Rosiak JM (2000) Hydroxyl radical reaction with melatonin molecule: A computational study. J. Pineal Res. 29 (2): 125–127. DOI: 10.1034/j.1600-079X.2000.290209.x.
272. Zang LY, Cosma G, Gardner H, Vallyathan V (1998) Scavenging of reactive oxygen species by melatonin. Biochim. Biophys. Acta - Gen. Subj. 1425 (3): 469–477. DOI: 10.1016/S0304-4165(98)00099-3.
273. Banerjee S, Joshi N, Mukherjee R, Singh PK, Baxi D, Ramachandran A V. (2017) Melatonin protects against chromium (VI) induced hepatic oxidative stress and toxicity: Duration dependent study with realistic dosage. Interdiscip. Toxicol. 10 (1): 20–29. DOI: 10.1515/intox-2017-0003.
274. Lee SH, Kim DH, Kuzmanov U, Gramolini AO (2021) Membrane proteomic profiling of the heart: Past, present, and future. Am. J. Physiol. - Hear. Circ. Physiol. 320 (1): H417–H423. DOI: 10.1152/AJPHEART.00659.2020/ASSET/IMAGES/LARGE/AJ-AHRT200097F003.JPEG.
275. Ceraulo L, Ferrugia M, Tesoriere L, Segreto S, Livrea MA, Turco Liveri V (1999) Interactions of melatonin with membrane models: Portioning of melatonin in AOT and lecithin reversed micelles. J. Pineal Res. 26 (2): 108–112. DOI: 10.1111/j.1600-079X.1999.tb00570.x.
276. Livrea MA, Tesoriere L, Arpa D, Morreale M (1997) Reaction of melatonin with lipoperoxyl radicals in phospholipid bilayers. Free Radic. Biol. Med. 23 (5): 706–711. DOI: 10.1016/S0891-5849(97)00018-X.
277. Reiter RJ, Acuña-Castroviejo D, Tan DX, Burkhardt S (2001) Free radical-mediated molecular damage: Mechanisms for the protective actions of melatonin in the central nervous system. Ann. N. Y. Acad. Sci. 939: 200–215. DOI: 10.1111/j.1749-6632.2001.tb03627.x.
278. Daniels WMU, Van Rensburg SJ, Van Zyl JM, Taljaard JJF (1998) Melatonin prevents β-amyloid-induced lipid peroxidation. J. Pineal Res. 24 (2): 78–82. DOI: 10.1111/j.1600-079X.1998.tb00370.x.
279. García JJ, Reiter RJ, Guerrero JM, Escames G, Yu BP, Oh CS, Muñoz-Hoyos A (1997) Melatonin prevents changes in microsomal membrane fluidity during induced lipid peroxidation. FEBS Lett. 408 (3): 297–300. DOI: 10.1016/S0014-5793(97)00447-X.
280. Aryafar T, Amini P, Rezapoor S, Shabeeb DD, Eleojo Musa A, Najafi M, Shirazi AI (2021) Modulation of radiation-induced NADPH oxidases in rat’s heart tissues by melatonin. J. Biomed. Phys. Eng. 11 (4): 465–472. DOI: 10.31661/JBPE.V0I0.1094.
281. Lv Y, Li T, Yang M, Su L, Zhu Z, Zhao S, Zeng W, Zheng Y (2021) Melatonin attenuates chromium (VI)-induced spermatogonial stem cell/progenitor mitophagy by restoration of METTL3-mediated RNA N6-methyladenosine modification. Front. Cell Dev. Biol. 9 (684398): 1–19. DOI: 10.3389/FCELL.2021.684398.
282. Seeger H, Mueck AO, Lippert TH (1997) Effect of melatonin and metabolites on copper-mediated oxidation of low density lipoprotein. Br. J. Clin. Pharmacol. 44 (3): 283–284. DOI: 10.1046/J.1365-2125.1997.00648.X.
283. Abuja PM, Liebmann P, Hayn M, Schauenstein K, Esterbauer H (1997) Antioxidant role of melatonin in lipid peroxidation of human LDL. FEBS Lett. 413 (2): 289–293. DOI: 10.1016/S0014-5793(97)00918-6.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


