Melatonin promotes gastric healing by modulating the components of matrix metalloproteinase signaling pathway: a novel scenario for gastric ulcer management
Melatonin protects gastric tissue by accelerating wound healing
Abstract
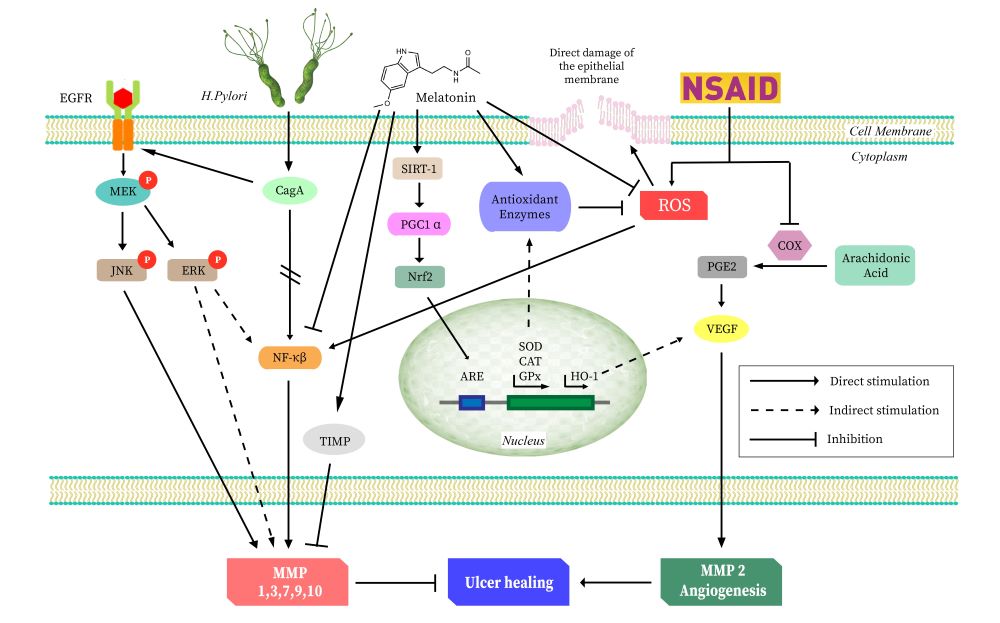
Over the past few decades, since the induction of antibiotics and proton pump inhibitors (PPI) as a therapeutic tool in controlling gastropathy, a substantial decline in the incidence of gastric ulcer and its related manifestations has been achieved globally. However, there are a lot of skeptics on the steady rise in the list of complications following long-term use of these drugs, especially in chronic and elderly patients. Hence, the search for a sustainable cure for these gastropathies has never actually ended; this let us consider that melatonin, an endogenous antioxidant, might have a utility in this respect. Although researchers have linked melatonin with accelerated post ulcerative wound healing, many of these studies have failed to identify the confounding factors and plausible healing mechanisms. In this review, we attempt to identify the underline mechanisms as to the protective effects of melatonin on a variety of gastropathies. Based on the evidence, we select the matrix metalloproteinases (MMPs) to be the main targets of melatonin. MMPs play a key role in maintaining the balance between extracellular matrix degradation and tissue remodeling, therefore, they act as the integral connection between the ulcer manifestation and healing. Thus, gastric ulceration occurs where this balance is disrupted. Melatonin can preserve this balance during the onset of gastric ulcers. In this review, we have also discussed the effects of melatonin on the different isoforms of MMPs and their roles in gastric ulceration, respectively. We hope that this will bestow us with a better understanding of the development of the gastric ulcer, as well as its cure.
References
2. Snowden FM (2008) Emerging and reemerging diseases: A historical perspective. Immunol. Rev. 225 (1): 9–26. DOI: 10.1111/j.1600-065X.2008.00677.x.
3. Prabhu V, Shivani A (2014) An overview of history, pathogenesis and treatment of perforated peptic ulcer disease with evaluation of prognostic scoring in adults. Ann. Med. Health Sci. Res. 4 (1): 22–29. DOI: 10.4103/2141-9248.126604.
4. Ballinger A, Smith G (2001) COX-2 inhibitors vs. NSAIDs in gastrointestinal damage and prevention. Expert Opin. Pharmacother. 2 (1): 31–40. DOI: 10.1517/14656566.2.1.31.
5. Huang JQ, Sridhar S, Hunt RH (2002) Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: A meta-analysis. Lancet 359 (9300): 14–22. DOI: 10.1016/S0140-6736(02)07273-2.
6. Müller-Quernheim J (2011) MMPs are regulatory enzymes in pathways of inflammatory disorders, tissue injury, malignancies and remodelling of the lung. T. Eur. Res. J. Eng. 38: 12–14. DOI: 10.1183/09031936.00079311.
7. Murphy G, Nagase H (2009) Progress in matrix metalloproteinase research. Mol. Aspects Med. 29 (5): 290–308. DOI: 10.1016/j.mam.2008.05.002.
8. Coussens LM, Fingleton B, Matrisian LM (2002) Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science 295 (5564): 2387–2392. DOI: 10.1126/science.1067100.
9. McCawley LJ, Matrisian LM (2001) Matrix metalloproteinases: They’re not just for matrix anymore! Curr. Opin. Cell Biol. 13 (5): 534–540. DOI: 10.1016/s0955-0674(00)00248-9.
10. Shahin M, Konturek JW, Pohle T, Schuppan D, Herbst H, Domschke W (2001) Remodeling of extracellular matrix in gastric ulceration. Microsc. Res. Tech. 53 (6): 396–408. DOI: 10.1002/jemt.1108.
11. Swarnakar S, Ganguly K, Kundu P, Banerjee A, Maity P, Sharma A V. (2005) Curcumin regulates expression and activity of matrix metalloproteinases 9 and 2 during prevention and healing of indomethacin-induced gastric ulcer. J. Biol. Chem. 280 (10): 9409–9415. DOI: 10.1074/jbc.M413398200.
12. Ganguly K, Maity P, Reiter RJ, Swarnakar S (2005) Effect of melatonin on secreted and induced matrix metalloproteinase-9 and -2 activity during prevention of indomethacin-induced gastric ulcer. J. Pineal Res. 39 (3): 307–315. DOI: 10.1111/j.1600-079X.2005.00250.x.
13. Lempinen M, Inkinen K, Wolff H, Ahonen J (2000) Matrix metalloproteinases 2 and 9 in indomethacin-lnduced rat gastric ulcer. Eur. Surg. Res. 32 (3): 169–176. DOI: 10.1159/000008759.
14. Redman J, Armstrong S, Ng KT (1983) Free-running activity rhythms in the rat: Entrainment by melatonin. Science 219 (4588): 1089–1091. DOI: 10.1126/science.6823571.
15. Galano A, Tan DX, Reiter RJ (2011) Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 51 (1): 1–16. DOI: 10.1111/j.1600-079X.2011.00916.x.
16. Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX, Reiter RJ (2014) Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 71 (16): 2997–3025. DOI: 10.1007/s00018-014-1579-2.
17. Messner M, Huether G, Lorf T, Ramadori G, Schwörer H (2001) Presence of melatonin in the human hepatobiliary-gastrointestinal tract. Life Sci. 69 (5): 543–551. DOI: 10.1016/s0024-3205(01)01143-2.
18. Reiter RJ, Tan D ‐X, Poeggeler B, Menendez‐Pelaez A, Chen L ‐D, Saarela S (1994) Melatonin As a Free Radical Scavenger: Implications for Aging and Age‐Related Diseases. Ann. N. Y. Acad. Sci. 31 (719): 1–12. DOI: 10.1111/j.1749-6632.1994.tb56817.x.
19. Pieri C, Marra M, Moroni F, Recchioni R, Marcheselli F (1994) Melatonin: A peroxyl radical scavenger more effective than vitamin E. Life Sci. 55 (15): 271–276. DOI: 10.1016/0024-3205(94)00666-0.
20. Majumder R, Datta M, Pal PK, Bhattacharjee B, Chattopadhyay A, Bandyopadhyay D (2019) Protective mechanisms of melatonin on caprine spleen injury induced by cadmium (Cd): an in vitro study. Mel. Res. 2 (3): 57–75. DOI: 10.32794/11250031.
21. Kusters JG, Van Vliet AHM, Kuipers EJ (2006) Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 19 (3): 449–490. DOI: 10.1128/CMR.00054-05.
22. Suerbaum S, Michetti P (2002) Helicobacter pylori Infection. N. Engl. J. Med. 347 (15): 1175–1186. DOI: 10.1056/NEJMra020542.
23. Ernst PB, Gold BD (2000) The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu. Rev. Microbiol. 54 : 615–640. DOI: 10.1146/annurev.micro.54.1.615.
24. Waskito LA, Salama NR, Yamaoka Y (2018) Pathogenesis of Helicobacter pylori infection. Helicobacter 23 : 1–6. DOI: 10.1111/hel.12516.
25. Konturek SJ, Konturek PC, Hartwich A, Hahn EG (2000) Helicobacter pylori infection and gastrin and cyclooxygenase expression in gastric and colorectal malignancies. Regul. Pept. 93 (1–3): 13–19. DOI: 10.1016/s0167-0115(00)00173-7.
26. Konturek JW (2003) Discovery by Jaworski of Helicobacter pylori and its pathogenetic role in peptic ulcer, gastritis and gastric cancer. J. Physiol. Pharmacol. 54 : 23–41. https://pubmed.ncbi.nlm.nih.gov/15075463.
27. Beall DP (1997) Classification and grading of gastritis: The updated Sydney system. Radiology 203 (2): 434. DOI: 10.1148/radiology.203.2.434.
28. Noach LA, Bosma NB, Jansen J, Hoek FJ, Van Deventer SJH, Tytgat GNJ (1994) Mucosal tumor necrosis factor-or, interleukin-1/3, and interleukin-8 production in patients with helicobacter pylori infection. Scand. J. Gastroenterol. 29 (5): 425–429. DOI: 10.3109/00365529409096833.
29. Denizot Y, Sobhani I, Rambaud JC, Lewin M, Thomas Y, Benveniste J (1990) Paf-acether synthesis by Helicobacter pylori. Gut 31 (11): 1242–1245. DOI: 10.1136/gut.31.11.1242.
30. Merchant JL (2005) Inflammation, atrophy, gastric cancer: Connecting the molecular dots. Gastroenterology 129 (3): 1079–1082. DOI: 10.1053/j.gastro.2005.07.038.
31. Wroblewski LE, Shen L, Ogden S, Romero-Gallo J, Lapierre LA, Israel DA, Turner JR, Peek RM (2009) Helicobacter pylori dysregulation of gastric epithelial tight junctions by urease-mediated myosin II activation. Gastroenterology 136 (1): 236–246. DOI: 10.1053/j.gastro.2008.10.011.
32. Yao X, Smolka AJ (2019) Gastric parietal cell physiology and Helicobacter pylori–induced disease. Gastroenterology 156 (8): 2158–2173. DOI: 10.1053/j.gastro.2019.02.036.
33. Meropol SB, Chan KA, Chen Z, Finkelstein JA, Hennessy S, Lautenbach E, Platt R, Schech SD, Shatin D, Metlay JP (2008) Adverse events associated with prolonged antibiotic use. Pharmacoepidemiol. Drug Saf. 17 (5): 523–532. DOI: 10.1002/pds.1547.
34. Laine L (2001) Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology 120 (3): 594–606. DOI: 10.1053/gast.2001.21907.
35. Musumba C, Pritchard DM, Pirmohamed M (2009) Review article: Cellular and molecular mechanisms of NSAID-induced peptic ulcers. Aliment Pharmacol. Ther. 30 (6): 517–531. DOI: 10.1111/j.1365-2036.2009.04086.x.
36. Frölich JC (1997) A classification of NSAIDs according to the relative inhibition of cyclooxygenase isoenzymes. Trends Pharmacol. Sci. 18 (1): 30–34. DOI: 10.1016/S0165-6147(96)01017-6.
37. Cashman JN (1996) The mechanisms of action of NSAIDs in analgesia. Drugs 52 : 13–23. DOI: 10.2165/00003495-199600525-00004.
38. Tripathi KD 2004. (2004) Essentials of Medical Pharmacology. Jaypee Brothers, Medical Publishers. 184–185 p. DOI: 10.5005/jp/books/12256.
39. Tanaka A, Araki H, Hase S, Komoike Y, Takeuchi K (2002) Up-regulation of COX-2 by inhibition of COX-1 in the rat: a key to NSAID-induced gastric injury. Aliment Pharmacol. Ther. 16 : 90–101. DOI: 10.1046/j.1365-2036.16.s2.22.x.
40. Langenbach R, Morham SG, Tiano HF, Loftin CD, Ghanayem BI, Chulada PC, Mahler JF, Lee CA, Goulding EH, Kluckman KD, Kim HS, Smithies O (1995) Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell 83 (3): 483–492. DOI: 10.1016/0092-8674(95)90126-4.
41. Takeuchi K, Takehara K, Ohuchi T (1996) Diethyldithiocarbamate, a superoxide dismutase inhibitor, reduces indomethacin-induced gastric lesions in rats. Digestion 57 (3): 201–209. DOI: 10.1159/000201341.
42. Wallace JL, McKnight W, Reuter BK, Vergnolle N (2000) NSAID-Induced gastric damage in rats: Requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology 119 (3): 706–714. DOI: 10.1053/gast.2000.16510.
43. Malfertheiner P, Kandulski A, Venerito M (2017) Proton-pump inhibitors: understanding the complications and risks. Nat. Rev. Gastroenterol. Hepatol. 14 (12): 697–710. DOI: 10.1038/nrgastro.2017.117.
44. Vu TH, Werb Z (2000) Matrix metalloproteinases: Effectors of development and normal physiology. Genes Dev. 14 : 2123–2133. DOI: 10.1101/gad.815400
45. Clark IM, Swingler TE, Sampieri CL, Edwards DR (2008) The regulation of matrix metalloproteinases and their inhibitors. Int. J. Biochem. Cell Biol. 40 (6–7): 1362–1378. DOI: 10.1016/j.biocel.2007.12.006.
46. Sharma AV, Ganguly K, Paul S, Maulik N, Swarnakar S (2012) Curcumin heals indomethacin-induced gastric ulceration by stimulation of angiogenesis and restitution of collagen fibers via vegf and mmp-2 mediated signaling. Antioxid. Redox Signal. 16 (4): 351–362. DOI: 10.1089/ars.2011.4232.
47. Parks WC (2006) Matrix Metalloproteinases. Encycl. Respir. Med. Four-Volume Set. (37): 18–25. DOI: 10.1016/B0-12-370879-6/00234-9.
48. Mori N, Sato H, Hayashibara T, Senba M, Geleziunas R, Wada A, Hirayama T, Yamamoto N (2003) Helicobacter pylori induces matrix metalloproteinase-9 through activation of nuclear factor κB. Gastroenterology 124 (4): 983–992. DOI: 10.1053/gast.2003.50152.
49. Ganguly K, Swarnakar S (2012) Chronic gastric ulceration causes matrix metalloproteinases-9 and -3 augmentation: Alleviation by melatonin. Biochimie 94 (12): 2687–2698. DOI: 10.1016/j.biochi.2012.08.004.
50. Ganguly K, Sharma AV, Reiter RJ, Swarnakar S (2010) Melatonin promotes angiogenesis during protection and healing of indomethacin-induced gastric ulcer: Role of matrix metaloproteinase-2. J. Pineal Res. 49 : 130–140. DOI: 10.1111/j.1600-079X.2010.00776.x.
51. Netzel-Arnett S, Sang QX, Moore WGI, Birkedal-Hansen H, Navre M, Van Wart HE (1993) Comparative sequence specificities of human 72-and 92-kDa gelatinases (type iv collagenases) and PUMP (Matrilysin). Biochemistry 32 (25): 6427–6432. DOI: 10.1021/bi00076a016.
52. Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, Borodovsky M, Rappuoli R, Covacci A (1996) cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. 93 (25): 14648–14653. DOI: 10.1073/pnas.93.25.14648.
53. Akopyants NS, Clifton SW, Kersulyte D, Crabtree JE, Youree BE, Reece CA, Bukanov NO, Drazek ES, Roe BA, Berg DE (1998) Analyses of the cag pathogenicity island of Helicobacter pylori. Mol. Microbiol. 28 (1): 37–53. DOI: 10.1046/j.1365-2958.1998.00770.x.
54. Nishikawa H, Hatakeyama M (2017) Sequence polymorphism and intrinsic structural disorder as related to pathobiological performance of the Helicobacter pylori CagA oncoprotein. Toxins (Basel). 9 (4): 136–150. DOI: 10.3390/toxins9040136.
55. Selbach M, Moese S, Hauck CR, Meyer TF, Backert S (2002) Src is the kinase of the Helicobacter pylori CagA protein in vitro and in vivo. J. Biol. Chem. 277 (9): 6775–6778. DOI: 10.1074/jbc.C100754200.
56. Ren S, Higashi H, Lu H, Azuma T, Hatakeyama M (2006) Structural basis and functional consequence of Helicobacter pylori cagA multimerization in cells. J. Biol. Chem. 281 (43): 32344–32352. DOI: 10.1074/jbc.M606172200.
57. Lamb A, Yang XD, Tsang YHN, Li JD, Higashi H, Hatakeyama M, Peek RM, Blanke SR, Chen LF (2009) Helicobacter pylori CagA activates NF-κB by targeting TAK1 for TRAF6-mediated Lys 63 ubiquitination. EMBO Rep. 10 (11): 1242–1249. DOI: 10.1038/embor.2009.210.
58. Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue JI, Cao Z, Matsumoto K (1999) The kinase TAK1 can activate the NIK-IκB as well as the MAP kinase cascade in the IL-1 signaling pathway. Nature 398 : 252–256. DOI: 10.1038/18465.
59. Krueger S, Hundertmark T, Kalinski T, Peitz U, Wex T, Malfertheiner P, Naumann M, Roessner A (2006) Helicobacter pylori encoding the pathogenicity island activates matrix metalloproteinase 1 in gastric epithelial cells via JNK and ERK. J. Biol. Chem. 281 (5): 2868–2875. DOI: 10.1074/jbc.M511053200
60. Crawford HC, Krishna US, Israel DA, Matrisian LM, Washington MK, Peek RM (2003) Helicobacter pylori strain-selective induction of matrix metalloproteinase-7 in vitro and within gastric mucosa. Gastroenterology 125 (4): 1125–1136. DOI: 10.1016/s0016-5085(03)01206-x.
61. Costa AM, Ferreira RM, Pinto-Ribeiro I, Sougleri IS, Oliveira MJ, Carreto L, Santos MA, Sgouras DN, Carneiro F, Leite M, Figueiredo C (2016) Helicobacter pylori activates matrix metalloproteinase 10 in gastric epithelial cells via EGFR and ERK-mediated pathways. J. Infect. Dis. 213 (11): 1767-76. DOI: 10.1093/infdis/jiw031.
62. Yan F, Cao H, Chaturvedi R, Krishna U, Hobbs SS, Dempsey PJ, Peek RM, Cover TL, Washington MK, Wilson KT, Polk DB (2009) Epidermal growth factor receptor activation protects gastric epithelial cells from Helicobacter pylori-induced apoptosis. Gastroenterology 136 (4): 1297–1307. DOI: 10.1053/j.gastro.2008.12.059.
63. Sierra JC, Asim M, Verriere TG, Piazuelo MB, Suarez G, Romero-Gallo J, Delgado AG, Wroblewski LE, Barry DP, Peek RM, Gobert AP, Wilson KT (2018) Epidermal growth factor receptor inhibition downregulates Helicobacter pylori-induced epithelial inflammatory responses, DNA damage and gastric carcinogenesis. Gut 67 (7): 1247–1260. DOI: 10.1136/gutjnl-2016-312888.
64. Lanas A, Carrera-Lasfuentes P, Arguedas Y, García S, Bujanda L, Calvet X, Ponce J, Perez-Aísa Á, Castro M, Muñoz M, Sostres C, García-Rodríguez LA (2015) Risk of upper and lower gastrointestinal bleeding in patients taking nonsteroidal anti-inflammatory drugs, antiplatelet agents, or anticoagulants. Clin. Gastroenterol. Hepatol. 13 (5): 906-12.e2. DOI: 10.1016/j.cgh.2014.11.007.
65. Wallace JL (1997) Nonsteroidal anti-inflammatory drugs and gastroenteropathy: The second hundred years. Gastroenterology 112 (3): 1000–1016. DOI: 10.1053/gast.1997.v112.pm9041264.
66. Konturek SJ, Brzozowski T, Stachura J, Dembinski A, Majka J (1994) Role of gastric blood flow, neutrophil infiltration, and mucosal cell proliferation in gastric adaptation to aspirin in the rat. Gut 35 (9): 1189–1196. DOI: 10.1136/gut.35.9.1189.
67. Cheng HC, Yang HB, Chang WL, Chen WY, Yeh YC, Sheu BS (2012) Expressions of MMPs and TIMP-1 in gastric ulcers may differentiate H. pylori-infected from NSAID-related ulcers. Sci. World J. 2012 . DOI: 10.1100/2012/539316.
68. Fu X, Kassim SY, Parks WC, Heinecke JW (2003) Hypochlorous acid generated by myeloperoxidase modifies adjacent tryptophan and glycine residues in the catalytic domain of matrix metalloproteinase-7 (matrilysin). An oxidative mechanism for restraining proteolytic activity during inflammation. J. Biol. Chem. 278 (31): 28403–28409. DOI: 10.1074/jbc.M304739200.
69. Miura T, Muraoka S, Fujimoto Y (2002) Lipid peroxidation induced by indomethacin with horseradish peroxidase and hydrogen peroxide: Involvement of indomethacin radicals. Biochem. Pharmacol. 63 (11): 2069–2074. DOI: 10.1016/s0006-2952(02)00995-4.
70. Nabavi SM, Nabavi SF, Sureda A, Xiao J, Dehpour AR, Shirooie S, Silva AS, Baldi A, Khan H, Daglia M (2019) Anti-inflammatory effects of melatonin: A mechanistic review. Crit. Rev. Food Sci. Nutr. 59 (sup1): S4–S16. DOI: 10.1080/10408398.2018.1487927.
71. Yoshikawa T, Naito Y, Kishi A, Tomii T, Kaneko T, Iinuma S, Ichikawa H, Yasuda M, Takahashi S, Kondo M (1993) Role of active oxygen, lipid peroxidation, and antioxidants in the pathogenesis of gastric mucosal injury induced by indomethacin in rats. Gut 34 (6): 732–737. DOI: 10.1136/gut.34.6.732.
72. Usardi MM, Franceschini J, Mandelli V, Daturi S, Mizzotti B (1974) Prostaglandins VIII: A proposed role for PGE2 in the genesis of stress-induced gastric ulcers. Prostaglandins 8 (1): 43–51. DOI: /10.1016/0090-6980(74)90035-5.
73. Jana S, Chatterjee K, Ray AK, Dasmahapatra P, Swarnakar S (2016) Regulation of matrix metalloproteinase-2 activity by COX-2-PGE2-pAKT axis promotes angiogenesisin Endometriosis. PLoS One 11 (10): 1–18. DOI: 10.1371/journal.pone.0163540.
74. Tarnawski, A. S., Ahluwalia, A., & Jones, M. K. (2014). Angiogenesis in gastric mucosa: an important component of gastric erosion and ulcer healing and its impairment in aging. J. gastroenterol. hepatol. 29 (Suppl 4): 112–123. DOI: 10.1111/jgh.12734.
75. Bradbury D, Clarke D, Seedhouse C, Corbettt L, Stocks J, Knox A (2005) Vascular endothelial growth factor induction by prostaglandin E2 in human airway smooth muscle cells is mediated by E prostanoid EP 2/EP4 receptors and SP-1 transcription factor binding sites. J. Biol. Chem. 280 (34): 29993–30000. DOI: 10.1074/jbc.M414530200.
76. Rodrigues M, Xin X, Jee K, Babapoor-Farrokhran S, Kashiwabuchi F, Ma T, Bhutto I, Hassan SJ, Daoud Y, Baranano D, Solomon S, Lutty G, Semenza GL, Montaner S, Sodhi A (2013) VEGF secreted by hypoxic Müller cells induces MMP-2 expression and activity in endothelial cells to promote retinal neovascularization in proliferative diabetic retinopathy. Diabetes 62 (11): 3863–3873. DOI: 10.2337/db13-0014.
77. Ganguly K, Kundu P, Banerjee A, Reiter RJ, Swarnakar S (2006) Hydrogen peroxide-mediated downregulation of matrix metalloprotease-2 in indomethacin-induced acute gastric ulceration is blocked by melatonin and other antioxidants. Free Radic. Biol. Med. 41 (6): 911–925. DOI: 10.1016/j.freeradbiomed.2006.04.022.
78. Morgan MJ, Liu ZG (2011) Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 21 (1): 103–115. DOI: 10.1038/cr.2010.178.
79. Brzozowski T, Konturek PC, Konturek SJ, Sliwowski Z, Pajdo R, Drozdowicz D, Ptak A, Hahn EG (2001) Classic NSAID and selective cyclooxygenase (COX)-1 and COX-2 inhibitors in healing of chronic gastric ulcers. Microsc. Res. Tech. 53 (5): 343–353. DOI: 10.1002/jemt.1102.
80. Menges M, Chan CC, Zeitz M, Stallmach A (2000) Higher concentration of matrix-metalloproteinase 1 (interstitial collagenase) in H. pylori-compared to NSAID-induced gastric ulcers. Z. Gastroenterol. 38 (11): 887–891. DOI: 10.1055/s-2000-10300.
81. Reiter RJ, Tan D, Osuna C, Gitto E (2000) Actions of melatonin in the reduction of oxidative stress. J. Biomed. Sci. 7 (6): 444–458. DOI: 10.1007/BF02253360.
82. Hardeland R, Reiter RJ, Poeggeler B, Tan DX (1993) The significance of the metabolism of the neurohormone melatonin: antioxidative protection and formation of bioactive substances. Neurosci. Biobehav. Rev. 17 (3): 347–357. DOI: 10.1016/s0149-7634(05)80016-8
83. Reiter RJ (1999) Oxidative damage to nuclear DNA: amelioration by melatonin. NEL Review. Neuro Endocrinol. Lett. 20 (3–4): 145-150. https://pubmed.ncbi.nlm.nih.gov/11462105.
84. Reiter RJ, Tan DX, Manchester LC, Qi W (2001) Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence. Cell Biochem. Biophys. 34 (2): 237–256. DOI: 10.1385/CBB:34:2:237.
85. Tan DX, Manchester LC, Reiter RJ, Qi WB, Karbownik M, Calvo JR (2000) Significance of melatonin in antioxidative defense system: reactions and products. Biol. Signals Recept. 9 (3–4): 137–159. DOI: 10.1159/000014635.
86. Urata Y, Honma S, Goto S, Todoroki S, Iida T, Cho S, Honma K, Kondo T (1999) Melatonin induces gamma-glutamylcysteine synthetase mediated by activator protein-1 in human vascular endothelial cells. Free Radic. Biol. Med. 27 (7–8): 838–847. DOI: 10.1016/s0891-5849(99)00131-8.
87. Harris ED (1992) Regulation of antioxidant enzymes. FASEB J. 6 (9): 2675–2683. DOI: 10.1096/fasebj.6.9.1612291.
88. Quijano C, Alvarez B, Gatti RM, Augusto O, Radi R (1997) Pathways of peroxynitrite oxidation of thiol groups. Biochem. J. 322 (Pt 1): 167–173. DOI: 10.1042/bj3220167.
89. Lopez-Gonzalez MA, Guerrero JM, Rojas F, Delgado F (2000) Ototoxicity caused by cisplatin is ameliorated by melatonin and other antioxidants. J. Pineal Res. 28 (2): 73–80. DOI: 10.1034/j.1600-079x.2001.280202.x.
90. Poeggeler B, Saarela S, Reiter RJ, Tan D ‐X, Chen L ‐D, Manchester LC, Barlow‐Walden LR (1994) Melatonin—a highly potent endogenous radical scavenger and electron donor: New aspects of the oxidation chemistry of this indole accessed in vitro. Ann. N. Y. Acad. Sci. 738 (1): 419–420. DOI: 10.1111/j.1749-6632.1994.tb21831.x.
91. Zang LY, Cosma G, Gardner H, Vallyathan V (1998) Scavenging of reactive oxygen species by melatonin. Biochim. Biophys. Acta 1425 (3): 469–477. DOI: 10.1016/s0304-4165(98)00099-3.
92. Marshall KA, Reiter RJ, Poeggeler B, Aruoma OI, Halliwell B (1996) Evaluation of the antioxidant activity of melatonin in vitro. Free Radic. Biol. Med. 21 (3): 307–315. DOI: 10.1016/0891-5849(96)00046-9.
93. Pick E, Keisari Y (1980) A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J. Immunol. Methods 38 (1–2): 161–170. DOI: 10.1016/0022-1759(80)90340-3.
94. Dellegar SM, Murphy SA, Bourne AE, DiCesare JC, Purser GH (1999) Identification of the factors affecting the rate of deactivation of hypochlorous acid by melatonin. Biochem. Biophys. Res. Commun. 257 (2): 431–439. DOI: 10.1006/bbrc.1999.0438. DOI: 10.1006/bbrc.1999.0438.
95. Chan T-Y, Tang P-L (1996) Characterization of the antioxidant effects of melatonin and related indoleamines in vitro. J. Pineal Res. 20 (4): 187–191. DOI: 10.1111/j.1600-079X.1996.tb00257.x.
96. Brzozowski T, Konturek PC, Konturek SJ, Pajdo R, Bielanski W, Brzozowska I, Stachura J, Hahn EG (1997) The role of melatonin and L-tryptophan in prevention of acute gastric lesions induced by stress, ethanol, ischemia, and aspirin. J. Pineal Res. 23 (2): 79–89. DOI: 10.1111/j.1600-079x.1997.tb00339.x.
97. Ahmed E, Anwar N, Galal O, El-sabahy M, Taha M (2017) Gastroprotective potential of melatonin versus melatonin loaded niosomes on gastric ulcer healing in rats. Comp. Clin. Path. 26 (1): 35–50. DOI: 10.1007/s00580-016-2344-8.
98. Konturek PC, Konturek SJ, Celinski K, Slomka M, Cichoz-Lach H, Bielanski W, Reiter RJ (2010) Role of melatonin in mucosal gastroprotection against aspirin-induced gastric lesions in humans. J. Pineal Res. 48 (4): 318–323. DOI: 10.1111/j.1600-079x.2010.00755.x.
99. Ahluwalia A, Brzozowska IM, Hoa N, Jones MK, Tarnawski AS 2018. (2018) Melatonin signaling in mitochondria extends beyond neurons and neuroprotection: Implications for angiogenesis and cardio/gastroprotection. Proc. Natl. Acad. Sci. 115 (9):E1942-E1943. DOI: 10.1073/pnas.1722131115.
100. Huether G (1993) The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia 49 (8): 665–670. DOI: 10.1007/BF01923948.
101. Huether G, Poeggeler B, Reimer A, George A (1992) Effect of tryptophan administration on circulating melatonin levels in chicks and rats: evidence for stimulation of melatonin synthesis and release in the gastrointestinal tract. Life Sci. 51 (12): 945–953. DOI: 10.1073/pnas.1722131115.
102. Shida CS, Castrucci AML, Lamy-Freund MT (1994) High melatonin solubility in aqueous medium. J. Pineal Res. 16 (4): 198–201. DOI: 10.1111/j.1600-079X.1994.tb00102.x.
103. Reiter RJ, Tan DX, Qi WB (1998) Suppression of oxygen toxicity by melatonin. Zhongguo Yao Li Xue Bao. 19 (6): 575–581. https://pubmed.ncbi.nlm.nih.gov/10437151.
104. Pal PK, Bhattacharjee B, Chattopadhyay A, Bandyopadhyay D (2019) Melatonin as an armament against non-steroidal anti-inflammatory drug induced gastric injury: An overview. Melatonin Res. 2 (1): 115–137. DOI: 10.32794/mr11250015.
105. Pal PK, Bhattacharjee B, Chattopadhyay A, Bandyopadhyay D (2019) Pleiotropic roles of melatonin against oxidative stress mediated tissue injury in the gastrointestinal tract: An overview. Melatonin Res. 2 (2): 158–184. DOI: 10.32794/mr11250027.
106. Celinski K, Konturek PC, Konturek SJ, Slomka M, Cichoz-Lach H, Brzozowski T, Bielanski W (2011) Effects of melatonin and tryptophan on healing of gastric and duodenal ulcers with Helicobacter pylori infection in humans. J. Physiol. Pharmacol. 62 (5): 521–526. https://pubmed.ncbi.nlm.nih.gov/22204799.
107. Konturek SJ, Konturek PC, Brzozowski T (2006) Melatonin in gastroprotection against stress-induced acute gastric lesions and in healing of chronic gastric ulcers. J. Physiol. Pharmacol. 57 : 51–66. https://pubmed.ncbi.nlm.nih.gov/17233075.
108. Brzozowska I, Strzalka M, Drozdowicz D, Konturek S, Brzozowski T (2014) Mechanisms of esophageal protection, gastroprotection and ulcer healing by melatonin. implications for the therapeutic use of melatonin in gastroesophageal reflux disease (GERD) and peptic ulcer disease. Curr. Pharm. Des. 20 (30): 4807–4815. DOI: 10.2174/1381612819666131119110258.
109. de Oliveira Torres JD, de Souza Pereira R (2010) Which is the best choice for gastroesophageal disorders: Melatonin or proton pump inhibitors?. World J. Gastrointest. Pharmacol. Ther. 1 (5) : 102-106. DOI: 10.4292/wjgpt.v1.i5.102
110. Celinski K, Konturek SJ, Konturek PC, Brzozowski T, Cichoz-Lach H, Slomka M, Malgorzata P, Bielanski W, Reiter RJ (2011) Melatonin or L-tryptophan accelerates healing of gastroduodenal ulcers in patients treated with omeprazole. J. Pineal Res. 50 (4): 389–394. DOI: 10.1111/j.1600-079X.2011.00855.x.
111. Ganguly K, Swarnakar S (2009) Induction of matrix metalloproteinase-9 and -3 in nonsteroidal anti-inflammatory drug-induced acute gastric ulcers in mice: regulation by melatonin. J. Pineal Res. 47 (1): 43–55. DOI: 10.1111/j.1600-079X.2009.00687.x.
112. Stanciu AE, Zamfir-Chiru-Anton A, Stanciu MM, Pantea-Stoian A, Nitipir C, Gheorghe DC (2020) Serum melatonin is inversely associated with matrix metalloproteinase-9 in oral squamous cell carcinoma. Oncol. Lett. 19 (4): 3011–3020. DOI: 10.3892/ol.2020.11392.
113. Paul S, Sharma AV, Mahapatra P Das, Bhattacharya P, Reiter RJ, Swarnakar S (2008) Role of melatonin in regulating matrix metalloproteinase-9 via tissue inhibitors of metalloproteinase-1 during protection against endometriosis. J. Pineal Res. 44 (4): 439–449. DOI: 10.1111/j.1600-079X.2007.00547.x.
114. Qin W, Lu W, Li H, Yuan X, Li B, Zhang Q, Xiu R (2012) Melatonin inhibits IL1β-induced MMP9 expression and activity in human umbilical vein endothelial cells by suppressing NF-κB activation. J. Endocrinol. 214 (2): 145–153. DOI: 10.1530/JOE-12-0147
115. Gutierrez-Cuesta J, Tajes M, Jiménez A, Coto-Montes A, Camins A, Pallàs M (2008) Evaluation of potential pro-survival pathways regulated by melatonin in a murine senescence model. J. Pineal Res. 45 (4): 497–505. DOI: 10.1111/j.1600-079X.2008.00626.x.
116. Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98 (1): 115–124. DOI: 10.1016/S0092-8674(00)80611-X.
117. Kim SJ, Kim JM, Shim SH, Chang HI (2014) Anthocyanins accelerate the healing of naproxen-induced gastric ulcer in rats by activating antioxidant enzymes via modulation of Nrf2. J. Funct. Foods 7 (1): 569–579. DOI: 10.1016/j.jff.2013.12.028.
118. Zhu H, Itoh K, Yamamoto M, Zweier JL, Li Y (2005) Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett. 579 (14): 3029–3036. DOI: 10.1016/j.febslet.2005.04.058.
119. Florczyk U, Jazwa A, Maleszewska M, Mendel M, Szade K, Kozakowska M, Grochot-Przeczek A, Viscardi M, Czauderna S, Bukowska-Strakova K, Kotlinowski J, Jozkowicz A, Loboda A, Dulak J (2014) Nrf2 regulates angiogenesis: effect on endothelial cells, bone marrow-derived proangiogenic cells and hind limb ischemia. Antioxid. Redox Signal 20 (11): 1693–1708. DOI: 10.1089/ars.2013.5219.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


