Protective mechanisms of melatonin on caprine spleen injury induced by cadmium (Cd): an in vitro study
Melatonin protects spleen tissue from cadmium toxicity
Abstract
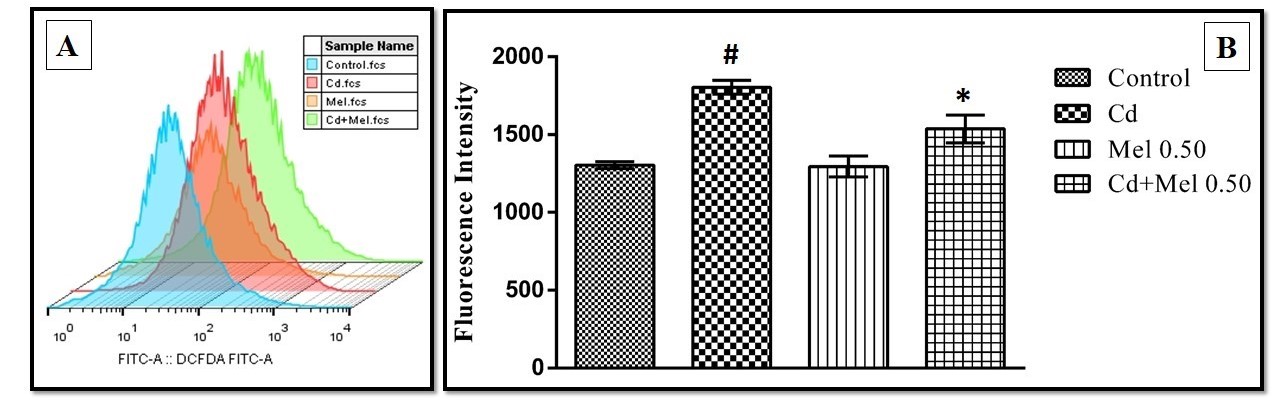
Current study explores the potential mechanisms of melatonin on cadmium-induced spleen tissue injury of goat. Spleen tissues were incubated with different concentrations (50, 100, 200, 400 and 600 µM) of cadmium acetate (Cd), respectively and the lipid peroxidation of the tissue was measured. It was found that Cd at the level of 400 µM induced maximum spleen damage among other concentrations. Thus, Cd 400 µM was selected to examine whether melatonin treatment can protect against this damage. The results showed that Cd increased the oxidative stress in the spleen tissue either by elevating pro-oxidant enzymes, or, by suppressing the variety of antioxidant enzymes and thus, to increase the intracellular reactive oxygen species (ROS). Melatonin treatment at the concentrations of 0.25, 0.5 and 1 mM significantly reduced all these alterations, respectively. At the level of cellular organelles, Cd caused mitochondrial morphological and functional injuries. These include mitochondrial surface distortion and inhibitions of glycolytic, Krebs cycle, and respiratory chain enzymes. Melatonin at a concentration of 0.5 mM almost completely preserved Cd induced mitochondrial pathological alterations. Cd pollution is a cause of serious health hazard world wide, particularly in the developing areas and currently, there is no specific remedy for Cd toxicities. The results suggest that melatonin is a promising therapeutic agent to combat Cd-induced oxidative stress and it deserves further investigation clinically.
References
2. Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, Opdenakker K, Nair AR, Munters E, Artois TJ, Nawrot T, Vangronsveld J, Smeets K (2010) Cadmium stress: An oxidative challenge. BioMetals 5: 927–940. DOI: 10.1007/s10534-010-9329-x.
3. Casalino E, Sblano C, Landriscina C (1997) Enzyme activity alteration by cadmium administration to rats: The possibility of iron involvement in lipid peroxidation. Arch Biochem. Biophys. 346 (2): 171–179. DOI: 10.1006/abbi.1997.0197.
4. Bauman JW, Liu J, Klaassen CD (1993) Production of metallothionein and heat-shock proteins in response to metals. Toxicol. Sci. 21 (1): 15–22. https://www.ncbi.nlm.nih.gov/ pubmed/8365580.
5. Wang X, Qu R, Liu J, Wei Z, Wang L, Yang S, Huang Q, Wang Z (2016) Effect of different carbon nanotubes on cadmium toxicity to Daphnia magna: The role of catalyst impurities and adsorption capacity. Environ. Pollut. 208 (B): 732–738. DOI: 10.1016/j.envpol.2015.10.053.
6. Qu RJ, Wang XH, Feng MB, Li Y, Liu HX, Wang LS, Wang ZY (2013) The toxicity of cadmium to three aquatic organisms (Photobacterium phosphoreum, Daphnia magna and Carassius auratus) under different pH levels. Ecotoxicol. Environ. Saf. 95 (1): 83–90. DOI: 10.1016/j.ecoenv.2013.05.020.
7. Qu R, Wang X, Liu Z, Yan Z, Wang Z (2013) Development of a model to predict the effect of water chemistry on the acute toxicity of cadmium to Photobacterium phosphoreum. J. Hazard. Mater. 262: 288–296. DOI: 10.1016/j.jhazmat.2013.08.039.
8. Godt J, Scheidig F, Grosse SC, Esche V, Brandenburg P, Reich A, Groneberg DA (2006) The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 1: 22. DOI: 10.1186/1745-6673-1-22.
9. Fasanya-Odewumi C, Latinwo LM, Ikediobi CO, Gilliard L, Sponholtz G, Nwoga J, Stino F, Hamilton N, Erdos GW (1998) The genotoxicity and cytotoxicity of dermally-administered cadmium: effects of dermal cadmium administration. Int. J. Mol. Med. 1 (6): 1001–1006. DOI: 10.3892/ijmm.1.6.1001.
10. Zalups RK, Ahmad S (2003) Molecular handling of cadmium in transporting epithelia. Toxicol. Appl. Pharmacol. 186 (3): 163–188. https://www.ncbi.nlm.nih.gov/pubmed/ 12620369.
11. Orłowski C, Piotrowski JK (2003) Biological levels of cadmium and zinc in the small intestine of non-occupationally exposed human subjects. Hum. Exp. Toxicol. 22 (2): 57–63. DOI: 10.1191/0960327103ht326oa.
12. Yiin SJ, Chern CL, Sheu JY, Lin TH (2001) Cadmium-induced liver, heart, and spleen lipid peroxidation in rats and protection by selenium. Biol. Trace Elem. Res. 78 (1–3): 219–230. DOI: 10.1385/BTER:78:1-3:219.
13. Pathak N, Khandelwal S (2007) Role of oxidative stress and apoptosis in cadmium induced thymic atrophy and splenomegaly in mice. Toxicol. Lett. 169 (2): 95–108. DOI: 10.1016/j.toxlet.2006.12.009.
14. Pivkin IV, Peng Z, Karniadakis GE, Buffet PA, Dao M, Suresh S (2016) Biomechanics of red blood cells in human spleen and consequences for physiology and disease. Proc. Natl. Acad. Sci. 113 (28): 7804–7809. DOI: 10.1073/pnas.1606751113.
15. Meldrum DR, Ayala A, Wang P, Ertel W, Chaudry IH (1991) Association between decreased splenic ATP levels and immunodepression: amelioration with ATP-MgCl2. Am. J. Physiol. Integr. Comp. Physiol. 261 (2): 351–357. DOI: 10.1152/ajpregu.1991. 261.2.R351.
16. Demenesku J, Mirkov I, Ninkov M, Popov Aleksandrov A, Zolotarevski L, Kataranovski D, Kataranovski M (2014) Acute cadmium administration to rats exerts both immunosuppressive and proinflammatory effects in spleen. Toxicology 256: 33–34. DOI: 10.1016/j.tox.2014.10.012.
17. Bhattacharjee B, Pal PK, Ghosh AK, Mishra S, Chattopadhyay A, Bandyopadhyay D (2019) Aqueous bark extract of Terminalia arjuna protects against cadmium-induced hepatic and cardiac injuries in male Wistar rats through antioxidative mechanisms. Food Chem. Toxicol. 124: 249–264. DOI: 10.1016/j.fct.2018.12.008.
18. Reina M, Castañeda-Arriaga R, Perez-Gonzalez A, Guzman-Lopez E, Tan D-X, Reiter R, Galano A (2018) A Computer-Assisted Systematic Search for Melatonin Derivatives with High Potential as Antioxidants. Melatonin Res. 1 (1): 27–58. DOI: https://doi.org/10.32794/mr11250003.
19. Maitra SK, Pal PK (2017) Melatonin rhythms in the pineal and non-pineal tissues and their physiological implications in subtropical fish. Biol. Rhythm Res. 48 (5): 757–776. DOI: 10.1080/09291016.2017.1345453.
20. Galano A, Tan DX, Reiter RJ (2011) Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 51 (1): 1–16. DOI: 10.1111/j.1600-079X.2011.00916.x.
21. Sanchez-Hidalgo M, de la Lastra CA, Carrascosa-Salmoral MP, Naranjo MC, Gomez-Corvera A, Caballero B, Guerrero JM (2009) Age-related changes in melatonin synthesis in rat extrapineal tissues. Exp. Gerontol. 44 (5): 328–334. DOI: 10.1016/j.exger.2009.02.002.
22. Pertsov SS (2006) Effect of melatonin on the thymus, adrenal glands, and spleen in rats during acute stress. Bull. Exp. Biol. Med. 141 (3): 292–295. https://www.ncbi.nlm.nih.gov/pubmed/17073142.
23. Bejarano I, Monllor F, Marchena AM, Ortiz A, Lozano G, Jiménez MI, Gaspar P, García JF, Pariente JA, Rodríguez AB, Espino J (2014) Exogenous melatonin supplementation prevents oxidative stress-evoked DNA damage in human spermatozoa. J. Pineal Res. 57 (3): 333–339. DOI: 10.1111/jpi.12172.
24. Chattopadhyay A, Biswas S, Bandyopadhyay D, Sarkar C, Datta AG (2003) Effect of isoproterenol on lipid peroxidation and antioxidant enzymes of myocardial tissue of mice and protection by quinidine. Mol. Cell. Biochem. 245 (1–2): 43–49. https://www.ncbi.nlm.nih.gov/pubmed/12708743.
25. Buege JA, Aust SD (1978) Microsomal Lipid Peroxidati.on. Methods Enzymol. 52: 302–310. https://www.ncbi.nlm.nih.gov/pubmed/672633.
26. Bandyopadhyay D, Ghosh G, Bandyopadhyay A, Reiter RJ (2004) Melatonin protects against piroxicam-induced gastric ulceration. J. Pineal Res. 36 (3): 195–203. https://www.ncbi.nlm.nih.gov/pubmed/15009511.
27. Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER (1990) Determination of Carbonyl Content in Oxidatively Modified Proteins. Methods Enzymol. 186: 464–478. https://www.ncbi.nlm.nih.gov/ pubmed/ 1978225.
28. Kjeld M (1972) An automated colorimetric method for the estimation of lactate dehydrogenase activity in serum. Scand. J. Clin. Lab. Invest. 29 (4): 421–425. https://www.ncbi.nlm.nih.gov/pubmed/21488412.
29. Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 25 (1): 192–205. https://www.ncbi.nlm.nih.gov/pubmed/4973948.
30. Wendell PL (2015) Measurement of oxidized glutathione and total glutathione in the perfused rat heart. Biochem. J. 117: 661–665. DOI: 10.1042/bj1170661.
31. Schafer FQ, Buettner GR (2001) Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 30 (11): 1191–1212. https://www.ncbi.nlm.nih.gov/pubmed/11368918.
32. Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 47 (3): 469–474. https://www.ncbi.nlm.nih.gov/pubmed/4215654.
33. Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 195 (1): 133–140. https://www.ncbi.nlm.nih.gov/pubmed/14938361.
34. Krohne-Ehrich G, Schirmer RH, Untucht-Grau R (2005) Glutathione Reductase from Human Erythrocytes. Eur. J. Biochem. 121 (2): 259–267. https://www.ncbi.nlm. nih. gov/pubmed/7032915.
35. Castro R, Piazzon MC, Noya M, Leiro M (2008) Isolation and molecular cloning of a fish myeloperoxidase. Mol. Immunol. 45: 428–437. DOI: 10.1016/j.molimm. 2007.05.028.
36. Greenlee L, Handler P (1964) Xanthine Oxidase. VI. Influence of pH on substrate specificity. J. Biol. Chem. 239 (4): 1090–1095. https://www.ncbi.nlm.nih.gov/ pubmed/14165912.
37. Mukherjee D, Ghosh AK, Dutta M, Mitra E, Mallick S, Saha B, Reiter RJ, Bandyopadhyay D (2015) Mitochondrial damage: protective actions of melatonin. J. Pineal Res. 58 (3): 275–290. DOI: 10.1111/jpi.12213.
38. Hare JF, Ching E, Attardi G (2005) Isolation, subunit composition, and site of synthesis of human cytochrome c oxidase. Biochemistry 19 (10): 2023–2030. https://www.ncbi.nlm.nih.gov/pubmed/6246917.
39. Noltmann EA, Gubler CJ, Kuby SA (1961) Glucose 6-phosphate dehydrogenase (Zwischenferment). I. Isolation of the crystalline enzyme from yeast. J. Biol. Chem. 236: 1225–1230. https://www.ncbi.nlm.nih.gov/pubmed/13729473.
40. Abdel-Hamid N, Ramadan M, Amgad S (2013) Glycoregulatory enzymes as early diagnostic markers during premalignant stage in hepatocellular carcinoma. Am. J. Cancer Prev. 1 (2): 14–19. DOI: 10.12691/ajcp-1-2-1.
41. Layzer RB, Rowland LP, Bank WJ (1969) Physical and kinetic properties of human phosphofructokinase from skeletal muscle and erythrocytes. J. Biol. Chem. 244 (14): 3823–3831. https://www.ncbi.nlm.nih.gov/pubmed/4241008.
42. Chretien D, Pourrier M, Bourgeron T, Séné M, Rötig A, Munnich A, Rustin P (1995) An improved spectrophotometric assay of pyruvate dehydrogenase in lactate dehydrogenase contaminated mitochondrial preparations from human skeletal muscle. Clin. Chim. Acta 240 (2): 129–136. https://www.ncbi.nlm.nih.gov/pubmed/8548923.
43. Shepherd D, Garland PB (2004) Citrate synthase from rat liver. Methods Enzymol.13: 11-16. DOI:10.1016/0076-6879(69)13006-2.
44. Duncan MJ, Fraenkel DG (1979) Alpha-ketoglutarate dehydrogenase mutant of Rhizobium meliloti. J. Bacteriol. 137 (1): 415–419. https://www.ncbi.nlm.nih.gov/ pubmed/762018.
45. Veeger C, DerVartanian DV, Zeylemaker WP (1969) [16] Succinate dehydrogenase. [EC 1.3.99.1 Succinate: (acceptor) oxidoreductase]. Methods Enzymol. 13: 85–90. DOI: https://doi.org/10.1016/0076-6879(69)13020-7.
46. Goyal N, Srivastava VML (1995) Oxidation and reduction of cytochrome c by mitochondrial enzymes of Setaria cervi. J. Helminthol. 69 (1): 13–17. https://www.ncbi.nlm.nih.gov/pubmed/7622786.
47. Bhogal RH, Curbishley SM, Weston CJ, Adams DH, Afford SC (2010) Reactive oxygen species mediate human hepatocyte injury during hypoxia/reoxygenation. Liver Transplant. 16 (11): 1303–1313. DOI: 10.1002/lt.22157.
48. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193 (1): 265–275. https://www.ncbi.nlm.nih.gov/ pubmed/14907713.
49. Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 329 (1–2): 23–38. https://www.ncbi.nlm.nih.gov/pubmed/12589963.
50. Poeggeler B, Saarela S, Reiter RJ, Tan DX, Chen LD, Manchester LC, Barlow-Walden LR. (1994) Melatonin- a highly potent endogenous radical scavenger and electron donor: new aspects of the oxidation chemistry of this indole accessed in vitro. Ann. N. Y. Acad. Sci. 738 (1): 419–420. https://www.ncbi.nlm.nih.gov/pubmed/7832450.
51. Messner B, Knoflach M, Seubert A, Ritsch A, Pfaller K, Henderson B, Shen YH, Zeller I, Willeit J, Laufer G, Wick G, Kiechl S, Bernhard D. (2009) Cadmium is a novel and independent risk factor for early atherosclerosis mechanisms and in vivo relevance. Arterioscler. Thromb. Vasc. Biol. 29 (9): 1392–1398. DOI: 10.1161/ ATVBAHA.109.190082.
52. Rana SVS, Verma S (1996) Protective effects of GSH, vitamin E, and selenium on lipid peroxidation in cadmium-fed rats. Biol. Trace Elem. Res. 51: 161–168. DOI: 10.1007/BF02785435.
53. Rikans LE, Hornbrook KR (1997) Lipid peroxidation, antioxidant protection and aging. Biochem. Biophys. Acta- Mol. Basis Dis. 1362 (2–3): 116–127. https://www.ncbi.nlm.nih.gov/pubmed/9540842.
54. Epstein FH, McCord JM (2010) Oxygen-derived free radicals in postischemic tissue injury. N. Engl. J. Med. 46: 2402–2406. DOI: 10.1056/NEJM198501173120305.
55. Chance B, Greenstein DS, Roughton FJW (1952) The mechanism of catalase action. I. Steady-state analysis. Arch. Biochem. Biophys. 37: 301–339. https://www.ncbi.nlm. nih.gov/pubmed/14953443.
56. Merra E, Calzaretti G, Bobba A, Storelli MM, Casalino E (2014) Antioxidant role of hydroxytyrosol on oxidative stress in cadmium-intoxicated rats: Different effect in spleen and testes. Drug Chem. Toxicol. 37 (4): 420–426. DOI: 10.3109/01480545. 2013.878950.
57. Gerson RJ, Shaikh ZA (1984) Differences in the uptake of cadmium and mercury by rat hepatocyte primary cultures. Role of a sulfhydryl carrier. Biochem. Pharmacol. 33 (2): 199–203. https://www.ncbi.nlm.nih.gov/pubmed/6704146.
58. Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ (2004) Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 36: 1–9. https://www.ncbi.nlm.nih.gov/pubmed/14675124.
59. Chung HY, Baek BS, Song SH, Kim MS, Huh JI, Shim KH, Kim KW, Lee KH (1997) Xanthine dehydrogenase/xanthine oxldase and oxidative stress. J. Am. Aging Assoc. 20 (3): 127–140. DOI: 10.1007/s11357-997-0012-2.
60. Battelli MG, Polito L, Bortolotti M, Bolognesi A (2016) Xanthine oxidoreductase-derived reactive species: Physiological and pathological effects. Oxid. Med. Cell. Longev. 548: 1–8. DOI: http://dx.doi.org/10.1155/2016/3527579.
61. Ramírez-Bajo MJ, de Atauri P, Ortega F, Westerhoff HV, Gelpí JL, Centelles JJ, Cascante M (2014) Effects of cadmium and mercury on the upper part of skeletal muscle glycolysis in mice. PLoS One 9 (1): e80018. DOI: 10.1371/journal.pone.0080018.
62. Nigam D, Shukla GS, Agarwal AK (1999) Glutathione depletion and oxidative damage in mitochondria following exposure to cadmium in rat liver and kidney. Toxicol. Lett. 106 (2–3): 151–157. https://www.ncbi.nlm.nih.gov/pubmed/10403659.
63. Tan DX, Manchester LC, Qin L, Reiter RJ (2016) Melatonin: A mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int. J. Mol. Sci. 17 (12): 2124. DOI: 10.3390/ijms17122124.
64. He C, Wang J, Zhang Z, Yang M, Li Y, Tian X, Ma T, Tao J, Zhu K, Song Y, Ji P, Liu G. (2016) Mitochondria synthesize melatonin to ameliorate its function and improve mice oocyte’s quality under in vitro conditions. Int. J. Mol. Sci. 17 (6): 939. DOI: 10.3390/ijms17060939.
65. Nijtmans LGJ, Klement P, Houštěk J, van den Bogert C (1995) Assembly of mitochondrial ATP synthase in cultured human cells: implications for mitochondrial diseases. BBA - Mol. Basis Dis. 1272 (3): 190–198. DOI: https://doi.org/10.1016/0925-4439(95)00087-9.
66. Capaldi RA, Aggeler R, Turina P, Wilkens S (1994) Coupling between catalytic sites and the proton channel in F 1 F 0 -type ATPases. Trends Biochem. Sci. 19 (7): 284–289. DOI: 10.1016/0968-0004(94)9006-X.
67. Nair AR, DeGheselle O, Smeets K, Van Kerkhove E, Cuypers A (2013) Cadmium-induced pathologies: Where is the oxidative balance lost (or not)? Int. J. Mol. Sci. 14 (3): 6116–6143. DOI: 10.3390/ijms14036116.
68. Müller L, Stacey NH (1988) Subcellular toxicity of low level cadmium in rats: Effect on cytochrome c oxidase. Toxicology 51 (1): 25–34. https://www.ncbi.nlm.nih.gov/ pubmed/2842893.
69. Kalyanaraman B, Darley-Usmar V, Davies KJ, Dennery PA, Forman HJ, Grisham MB, Mann GE, Moore K, Roberts LJ 2nd, Ischiropoulos H. (2012) Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic. Biol. Med. 52 (1): 1–6. DOI: 10.1016/j.freeradbiomed.2011.09.030.
70. Wang X, Fang H, Huang Z, Shang W, Hou T, Cheng A, Cheng H. (2013) Imaging ROS signaling in cells and animals. J. Mol. Med. 91 (8): 917–927. DOI: 10.1007/ s00109-013-1067-4.
71. Pal PK, Bhattacharjee B, Chattopadhyay A, Bandyopadhyay D (2019) Pleiotropic roles of melatonin against oxidative stress mediated tissue injury in the gastrointestinal tract: An overview. Melatonin Res. 2 (2): 158-184. DOI: https://doi.org/10.32794/ mr11250027.
72. Mitra E, Bhattacharjee B, Pal PK, Ghosh AK, Mishra S, Chattopadhyay A, Bandyopadhyay D (2019) Melatonin protects against cadmium-induced oxidative damage in different tissues of rat: a mechanistic insight. Melatonin Res. 2 (2): 1-21. DOI: https://doi.org/10.32794/mr11250018.
73. Tan DX, Reiter RJ (2019) Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2 (1): 44-66. DOI: https://doi.org/ 10.32794/mr11250011.
74. Pal PK, Bhattacharjee B, Ghosh AK, Chattopadhyay A, Bandyopadhyay D (2018) Adrenaline induced disruption of endogenous melatoninergic system, antioxidant and inflammatory responses in the gastrointestinal tissues of male Wistar rat: an in vitro study. Melatonin Res. 1 (1): 109–131. DOI: 10.32794/mr11250007.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


