Differential effects of melatonin on adipose tissues under normoestrogenic and estrogen-deficient conditions in rats
Melatonin effects on white and brown adipose tissues in rats
Abstract
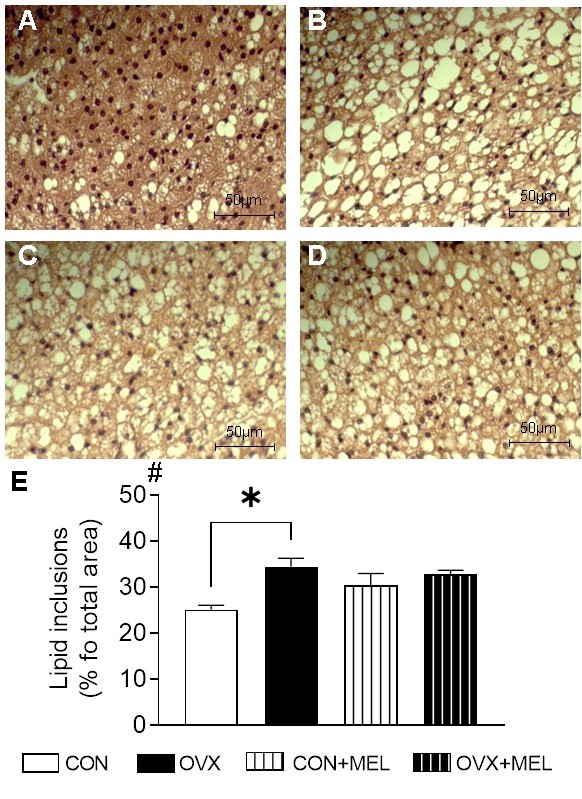
In post-menopause, oxidative stress due to the decline of natural antioxidants increases the susceptibility to metabolic syndromes (MetS). Estrogen and melatonin (MEL) share antioxidant properties; however, few studies have reported the impact of estrogen deficiency and MEL treatment on morphology, redox status, and antioxidant defense capacity of diverse adipose tissue (AT) subtypes. To investigate this issue, MEL was administered to ovariectomized (OVX) rats and sham-operated rats for 16 weeks (10 mg/kg). The adipocyte morphology, oxidative stress parameters and antioxidant enzyme activity were evaluated in the visceral retroperitoneal adipose tissue (rVAT), subcutaneous inguinal adipose tissue (iSAT) and brown adipose tissue (BAT). In OVX rats, MEL treatment suppressed rVAT hypertrophy and increased the prevalence of small adipocytes in iSAT, suggesting a better lipid distribution among ATs. MEL treatment increased glutathione reductase and glucose-6-phosphate dehydrogenase activity in iSAT; therefore, restored the glutathione level. In rVAT, MEL increased glutathione peroxidase and glutathione reductase activity. MEL minimized the risks for the development of metabolic abnormalities due to estrogen deficiency. However, under normoestrogenic condition, MEL decreased plasma estradiol levels and uterine mass, raising the concerning of its effect on reproductive functions.
References
2. Lopez-Gonzalez MA, Calvo JR, Segura JJ, Guerrero JM (1993) Characterization of melatonin binding sites in human peripheral blood neutrophils. Biotechnol. Ther. 4 (3-4): 253-262. https://www.ncbi.nlm.nih.gov/pubmed/8292973.
3. Faillace MP, Cutrera R, Sarmiento MI, Rosenstein RE (1995) Evidence for local synthesis of melatonin in golden hamster retina. Neuroreport 6 (15): 2093-2095. https://www.ncbi.nlm.nih.gov/pubmed/8580448.
4. Tan, DX, Manchester LC, Reiter RJ, Qi WB, Zhang M, Weintraub ST, Cabrera J, Sainz RM, Mayo JC, (1999) Identification of highly elevated levels of melatonin in bone marrow: its origin and significance. Biochim. Biophys. Acta 1472 (1-2): 206-214. https://www.ncbi.nlm.nih.gov/pubmed/10572942.
5. Lardone PJ, Carrillo-Vico A, Molinero P, Rubio A, Guerrero JM (2009) A novel interplay between membrane and nuclear melatonin receptors in human lymphocytes: significance in IL-2 production. Cell Mol. Life Sci. 66 (3): 516-525. https://www.ncbi.nlm.nih.gov/pubmed/19099187.
6. Conti A, Conconi S, Hertens E, Skwarlo-Sonta K, Markowska M, Maestroni JM (2000) Evidence for melatonin synthesis in mouse and human bone marrow cells. J. Pineal Res. 28 (4): 193-202. https://www.ncbi.nlm.nih.gov/pubmed/10831154.
7. Huether G, Poeggeler B, Reimer A, George A (1992) Effect of tryptophan administration on circulating melatonin levels in chicks and rats: evidence for stimulation of melatonin synthesis and release in the gastrointestinal tract. Life Sci. 51 (12): 945-953. https://www.ncbi.nlm.nih.gov/pubmed/1518369.
8. Roy D, Belsham DD (2002) Melatonin receptor activation regulates GnRH gene expression and secretion in GT1-7 GnRH neurons. Signal transduction mechanisms. J. Biol. Chem. 277 (1): 251-258. https://www.ncbi.nlm.nih.gov/pubmed/11684691.
9. Reiter RJ, Tamura H, Tan DX, Xu XY (2014) Melatonin and the circadian system: contributions to successful female reproduction. Fertil. Steril. 102 (2): 321-328. https://www.ncbi.nlm.nih.gov/pubmed/24996495.
10. Lobo RA (2008) Metabolic syndrome after menopause and the role of hormones. Maturitas 60 (1): 10-18. https://www.ncbi.nlm.nih.gov/pubmed/18407440.
11. Cagnacci A,, Arangino S, Angiolucci M, Melis GB, Tarquini R, Renzi A, Volpe A (2000) Different circulatory response to melatonin in postmenopausal women without and with hormone replacement therapy J. Pineal Res. 29 (3): 152-158. https://www.ncbi.nlm.nih.gov/pubmed/11034112.
12. Greendale GA, Witt-Enderby P, Karlamangla AS, Munmun F, Crawford S, Huang MH, Santoro N (2020) Melatonin patterns and levels during the human menstrual cycle and after menopause. J. Endocr. Soc. 4 (11): bvaa115. https://www.ncbi.nlm.nih.gov/pubmed/33094207.
13. Lima FB, Machado UF, Bartol I, Seraphim PM, Sumida DH, Moraes SM, Hell NS, Okamoto MM, Saad MJ, Carvalho CR, Cipolla-Neto J (1998) Pinealectomy causes glucose intolerance and decreases adipose cell responsiveness to insulin in rats. Am. J. Physiol. 275 (6) :E934-941. https://www.ncbi.nlm.nih.gov/pubmed/9843734.
14. Bellipanni G, Bianchi P, Pierpaoli W, Bulian D, Ilyia E (2001) Effects of melatonin in perimenopausal and menopausal women: a randomized and placebo controlled study. Exp. Gerontol. 36 (2): 297-310. https://www.ncbi.nlm.nih.gov/pubmed/11226744.
15. Chojnacki C, Kaczka A, Gasiorowska A, Fichna J, Chojnacki J, Brzozowski T (2018) The effect of long-term melatonin supplementation on psychosomatic disorders in postmenopausal women. J. Physiol. Pharmacol. 69 (2): 297-304. https://www.ncbi.nlm.nih.gov/pubmed/30045006.
16. Delpino FM, Figueiredo LM (2021) Melatonin supplementation and anthropometric indicators of obesity: A systematic review and meta-analysis. Nutrition 91-92:111399. https://www.ncbi.nlm.nih.gov/pubmed/34626955.
17. Genario R, Cipolla-Neto J, Bueno AA, Santos HO (2021) Melatonin supplementation in the management of obesity and obesity-associated disorders: A review of physiological mechanisms and clinical applications. Pharmacol. Res. 163: 105254. https://www.ncbi.nlm.nih.gov/pubmed/33080320.
18. Jimenez-Aranda A, Fernandez-Vazquez G, Campos D, Tassi M, Velasco-Perez L, Tan DX, Reiter RJ, Agil A (2013) Melatonin induces browning of inguinal white adipose tissue in Zucker diabetic fatty rats. J. Pineal Res. 55 (4): 416-423. https://www.ncbi.nlm.nih.gov/pubmed/24007241.
19. Salagre D, Navarro-Alarcón M, Villalón-Mir M, Alcázar-Navarrete B, Gómez-Moreno G, Tamimi F, Agil A (2024) Chronic melatonin treatment improves obesity by inducing uncoupling of skeletal muscle SERCA-SLN mediated by CaMKII/AMPK/PGC1α pathway and mitochondrial biogenesis in female and male Zücker diabetic fatty rats. Biomed. Pharmacother. 172: 116314. https://pubmed.ncbi.nlm.nih.gov/38387135.
20. Asarian L, Geary N (2002) Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm. Behav. 42 (4): 461-471. https://www.ncbi.nlm.nih.gov/pubmed/12488112.
21. Baxi D, Singh PK, Vachhrajani K, Ramachandran AV (2012) Melatonin supplementation therapy as a potent alternative to ERT in ovariectomized rats. Climacteric 15 (4): 382-392. https://www.ncbi.nlm.nih.gov/pubmed/22185471.
22. Finan B, Yang B, Ottaway N, Stemmer K, MüllerTD, Yi XC, Habegger K, Schriever S C, García-CA, Kabra DG (2012) Targeted estrogen delivery reverses the metabolic syndrome. Nat. Med. 18 (12): 1847-1856. https://www.ncbi.nlm.nih.gov/pubmed/23142820.
23. Mahboobifard F, Pourgholami MH, Jorjani M, Dargahi L, Amiri M, Sadeghi S, Tehrani FR (2022) Estrogen as a key regulator of energy homeostasis and metabolic health. Biomed. Pharmacoter. 156: 113808. https://www.ncbi.nlm.nih.gov/pubmed/36252357.
24. Xu Y, López M (2018) Central regulation of energy metabolism by estrogens. Mol. Metab. 15: 104-115. https://www.ncbi.nlm.nih.gov/pubmed/29886181.
25. Amstrup AK, Sikjaer T, Pedersen, SB, Heickendorff L, Mosekilde L, Rejnmark L (2016) Reduced fat mass and increased lean mass in response to 1 year of melatonin treatment in postmenopausal women: A randomized placebo-controlled trial. Clin. Endocrinol. 84 (3): 342-347. https://www.ncbi.nlm.nih.gov/pubmed/26352863.
26. Hsu LW, Chien YW (2023) Effects of melatonin supplementation on lipid metabolism and body fat accumulation in ovariectomized rats. Nutrients 15 (12): 2800. https://www.ncbi.nlm.nih.gov/pubmed/37375706.
27. Sanchez-Mateos S, Alonso-Gonzalez C, Gonzalez A, Martinez-Campa CM, Mediavilla MD, Cos S, Sanchez-Barcelo EJ, et al. (2007) Melatonin and estradiol effects on food intake, body weight, and leptin in ovariectomized rats. Maturitas 58 (1): 91-101. https://www.ncbi.nlm.nih.gov/pubmed/17706901.
28. Hermoso, DAM, Shimada LBC, Gilglioni EH, Constantin J, Mito MS, Hermoso APM, Salgueiro-Pagadigorria CL, Iwamoto ELI. (2016) Melatonin protects female rats against steatosis and liver oxidative stress induced by oestrogen deficiency. Life Sci. 157: 178-186. https://www.ncbi.nlm.nih.gov/pubmed/27262788.
29. Hoffstedt J, Arner E, Wahrenberg H, Andersson DP, Qvisth V, Lofgren P, Ryden M, Thorne A, Wiren M, Palmer M, Thorell A, Toft E, Arner P (2010) Regional impact of adipose tissue morphology on the metabolic profile in morbid obesity. Diabetologia 53 (12): 2496-2503. https://www.ncbi.nlm.nih.gov/pubmed/20830466.
30. Zhang M, Hu T, Zhang S, Zhou L (2015) Associations of different adipose tissue depots with insulin resistance: A systematic review and meta-analysis of observational studies. Sci. Rep. 5:18495. https://www.ncbi.nlm.nih.gov/pubmed/26686961.
31. Fang L, Guo F, Zhou L, Stahl R, Grams J (2015) The cell size and distribution of adipocytes from subcutaneous and visceral fat is associated with type 2 diabetes mellitus in humans. Adipocyte 4 (4): 273-279. https://www.ncbi.nlm.nih.gov/pubmed/26451283.
32. Le Gouic S, Atgié C, Viguerie-Bascands N, Hanoun N, Larrouy D, Ambid L, Raimbault S, Ricquier D, Delagrange P, Guardiola-Lemaitre B, Pénicaud L, Casteilla L (1997) Characterization of a melatonin binding site in Siberian hamster brown adipose tissue. Eur. J. Pharmacol. 339 (2-3): 271-278. https://www.ncbi.nlm.nih.gov/pubmed/9473145.
33. Lovejoy JC, Sainsbury A, Stock Conference Working G (2009) Sex differences in obesity and the regulation of energy homeostasis. Obes. Rev. 10 (2): 154-167. https://www.ncbi.nlm.nih.gov/pubmed/19021872.
34. Macotela Y, Boucher J, Tran TT, Kahn CR (2009) Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes 58 (4): 803-812. https://www.ncbi.nlm.nih.gov/pubmed/19136652.
35. Abildgaard J, Danielsen ER, Dorph E, Thomsen C, Juul A, Ewertsen C, Pedersen BK, Pedersen AT, Ploug T, Lindegaard B (2021) Changes in abdominal subcutaneous adipose tissue phenotype following menopause is associated with increased visceral fat mass. Sci. Rep. 11 (1): 14750. ://www.ncbi.nlm.nih.gov/pubmed/34285301.
36. Ahmed, Kamble PG, Hetty S, Fanni G, Milica M, Sarsenbayeva A, Kristófi R, Svensson MK, Pereira MJ, Eriksson JW (2022) Role of estrogen and its receptors in adipose tissue glucose metabolism in pre- and postmenopausal women. J. Clin. Endocrinol. Met. 107 (5): e1879-e1889. https://www.ncbi.nlm.nih.gov/pubmed/35084504.
37. Castro JP, Grune T, Speckmann B (2016) The two faces of reactive oxygen species (ROS) in adipocyte function and dysfunction. Biol. Chem. 397 (8): 709-724. https://www.ncbi.nlm.nih.gov/pubmed/27031218.
38. Murdolo G, Piroddi M, Luchetti F, Tortoioli C, Canonico B, Zerbinati C, Galli F, Iuliano L (2013) Oxidative stress and lipid peroxidation by-products at the crossroad between adipose organ dysregulation and obesity-linked insulin resistance. Biochimie 95 (3): 585-594. https://www.ncbi.nlm.nih.gov/pubmed/23274128.
39. Becker BN, Himmelfarb J, Henrich WL, Hakim RM (1997) Reassessing the cardiac risk profile in chronic hemodialysis patients: a hypothesis on the role of oxidant stress and other non-traditional cardiac risk factors. J. Am. Soc. Nephrol. 8 (3): 475-486. https://www.ncbi.nlm.nih.gov/pubmed/9071717.
40. Doshi SB, Agarwal A (2013) The role of oxidative stress in menopause. J. Midlife Health 4 (3): 140-146. https://www.ncbi.nlm.nih.gov/pubmed/24672185.
41. Behl C, Skutella T, Lezoualc'h F, Post A, Widmann M, Newton CJ, Holsboer F (1997) Neuroprotection against oxidative stress by estrogens: structure-activity relationship. Mol. Pharmacol. 51 (4): 535-541. https://www.ncbi.nlm.nih.gov/pubmed/9106616.
42. Borrás C, Gambini J, Gomez-Cabrera MC, Sastre J, Pallardo FV, Mann GE, Vina J (2005) 17beta-oestradiol up-regulates longevity-related, antioxidant enzyme expression via the ERK1 and ERK2[MAPK]/NFkappaB cascade. Aging cell 4 (3): 113-118. https://www.ncbi.nlm.nih.gov/pubmed/15924567.
43. Garcia-Ruiz C, Fernandez-Checa JC (2018) Mitochondrial oxidative stress and antioxidants balance in fatty liver disease. Hepatol. Commun. 2 (12): 1425-1439. https://www.ncbi.nlm.nih.gov/pubmed/30556032.
44. Suzen S, Atayik MC, Sirinzade H, Entezari B, Gurer-Orhan H, Çakatay U (2022) Melatonin and redox homeostasis. Melatonin Res. 5 (3): 304-324.
45. Reiter RJ, Tan DX, Terron MP, Flores LJ, Czarnocki Z (2007) Melatonin and its metabolites: new findings regarding their production and their radical scavenging actions. Acta Biochim. Pol. 54 (1): 1-9. https://www.ncbi.nlm.nih.gov/pubmed/17351668.
46. García JJ, Lopez-Pingarron L, Almeida-Souza P, Tres A, Escudero P, Garcia-Gil FA, Tan DX, Reiter RJ, Ramirez JM, Bernal-Perez M (2014) Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: A review. J. Pineal Res. 56 (3): 225-237. https://www.ncbi.nlm.nih.gov/pubmed/24571249.
47. Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226 (1): 497-509. https://www.ncbi.nlm.nih.gov/pubmed/13428781.
48. Latimer GW (2016) Official methods of analysis of AOAC International 20 Ed.
49. Marcondes FK, Biachi FJ, Tanno AP (2002) Determination of the estrous cycle phases of rats some helpful considerations. Braz. J. Biol. 62: 609-614.
50. Smith MS, Freeman ME, Neill JD (1975) The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 96 (1): 219-226. https://www.ncbi.nlm.nih.gov/pubmed/1167352.
51. Friedewald W, Levy Ra, Fredrickson D (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18: 499-502.
52. Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95 (2): 351-358. https://www.ncbi.nlm.nih.gov/pubmed/36810.
53. Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem. 25: 192-205. https://doi.org/10.1016/0003-2697(68)90092-4.
54. Hissin PJ, Hilf R (1976) A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 74 (1): 214-226. Https://www.ncbi.nlm.nih.gov/pubmed/962076.
55. Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J .Biochem. 47 (3): 469-474. https://www.ncbi.nlm.nih.gov/pubmed/4215654.
56. Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 70 (1): 158-169. https://www.ncbi.nlm.nih.gov/pubmed/6066618.
57. Mize CE, Langdon RG (1962) Hepatic glutathione reductase. I. Purification and general kinetic properties. J. Biol. Chem. 237:1589-1595. https://www.ncbi.nlm.nih.gov/pubmed/14474846.
58. Aebi H, Wyss SR, Scherz B, Skvaril F (1974) Heterogeneity of erythrocyte catalase II. Isolation and characterization of normal and variant erythrocyte catalase and their subunits. Eur. J. Biochem. 48 (1): 137-145. https://www.ncbi.nlm.nih.gov/pubmed/4141308.
59. Tian WN, Pignatare JN, Stanton RC (1994) Signal transduction proteins that associate with the platelet-derived growth factor (PDGF) receptor mediate the PDGF-induced release of glucose-6-phosphate dehydrogenase from permeabilized cells. J. Biol. Chem. 269 (20): 14798-14805.
60. Kaur J (2014) A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014: 943162. https://www.ncbi.nlm.nih.gov/pubmed/24711954.
61. Campos LB, Gilglioni EH, Garcia RF, Brito MN, Natali MR, Ishii-Iwamoto EL, Salgueiro-Pagadigorria CL (2012) Cimicifuga racemosa impairs fatty acid beta-oxidation and induces oxidative stress in livers of ovariectomized rats with renovascular hypertension. Free Radic. Biol. Med. 53 (4):.680-689. https://www.ncbi.nlm.nih.gov/pubmed/22684021.
62. Gilglioni EH, Campos LB, Oliveira MC, Garcia RF, Ambiel CR, Buzzo AJ, Ishii-Iwamoto E, Salgueiro-Pagadigorria CL (2013) Beneficial effects of tibolone on blood pressure and liver redox status in ovariectomized rats with renovascular hypertension. J. Geronto..A Biol. Sc.i Med. Sci. 68 (5): 510-520. https://www.ncbi.nlm.nih.gov/pubmed/23089337.
63. Abbas AM, Elsamanoudy AZ (2011) Effects of 17beta-estradiol and antioxidant administration on oxidative stress and insulin resistance in ovariectomized rats. Can. J. Physiol. Pharmacol. 89 (7): 497-504. https://www.ncbi.nlm.nih.gov/pubmed/21812527.
64. Camporez JP, Jornayvaz FR, Lee HY, Kanda S, Guigni BA, Kahn M, Samuel V, Carvalho CRO, Petersen KF, Jurczak MJ, Shulman GI (2013) Cellular mechanism by which estradiol protects female ovariectomized mice from high-fat diet-induced hepatic and muscle insulin resistance. Endocrinology 154 (3): 1021-1028. https://www.ncbi.nlm.nih.gov/pubmed/23364948.
65. Park SK, Park SK, Harlow SD, Zheng H, Karvonen-Gutierrez C, Thurston RC, Ruppert K, Janssen I, Randolph Jr JF (2017) Association between changes in oestradiol and follicle-stimulating hormone levels during the menopausal transition and risk of diabetes. Diabet. Med. 34 (4): 531-538. https://www.ncbi.nlm.nih.gov/pubmed/27973745.
66. LeBlanc ES, Kapphahn K, Hedlin H, Desai M, Parikh NI, Liu S, Parker DR, Anderson M, Aroda V, Sullivan S, Woods NF, Waring ME, Lewis CE, Stefanick M (2017) Reproductive history and risk of type 2 diabetes mellitus in postmenopausal women: findings from the Women's Health Initiative. Menopause 24 (1): 64-72. https://www.ncbi.nlm.nih.gov/pubmed/27465714.
67. Zidon TM, Padilla J, Fritsche KL, Welly RJ, McCabe LT, Stricklin OE, Frank A, Park Y, Clegg DJ, Lubahn DB, Kanaley JA, Vieira-Potter VJ (2020) Effects of ERbeta and ERalpha on OVX-induced changes in adiposity and insulin resistance. J. Endocrinol. 245 (1): 165-178. https://www.ncbi.nlm.nih.gov/pubmed/32053493.
68. Liu A, McLaughlin T, Liu T, Sherman A, Yee G, Abbasi F, Lamendola C, et al. (2009) Differential intra-abdominal adipose tissue profiling in obese, insulin-resistant women. Obes Surg. 19: 1564-1573.
69. Tandon P, Wafer R, Minchin JEN. Adipose morphology and metabolic disease. J. Exp. Biol. 2018: 221.
70. Imhoff BR, Hansen JM (2011) Differential redox potential profiles during adipogenesis and osteogenesis. Cell Mol. Biol. Lett. 16 (1): 149-161. https://www.ncbi.nlm.nih.gov/pubmed/21225471.
71. Masschelin PM, Cox AR, Chernis N, Hartig SM (2019) The impact of oxidative stress on adipose tissue energy balance. Front. Physiol. 10: 1638. https://www.ncbi.nlm.nih.gov/pubmed/32038305.
72. Jarukamjorn K, Jearapong N, Pimson C, & Chatuphonprasert W (2016) A high-fat, high-fructose diet induces antioxidant imbalance and increases the risk and progression of nonalcoholic fatty liver disease in mice. Scientifica (Cairo) 2016: 5029414. https://www.ncbi.nlm.nih.gov/pubmed/27019761.
73. Miller CG, Holmgren A, Arner ESJ, Schmidt EE (2018) NADPH-dependent and -independent disulfide reductase systems. Free Radic. Biol. Med. 127: 248-261. https://www.ncbi.nlm.nih.gov/pubmed/29609022.
74. Wang X, Ma Y, Huang C, Wan Q, Li N, Bi Y (2008) Glucose-6-phosphate dehydrogenase plays a central role in modulating reduced glutathione levels in reed callus under salt stress. Planta 227 (3): 611-623. https://www.ncbi.nlm.nih.gov/pubmed/17952457.
75. Cui J, Shen Y, Li R (2013) Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol. Med. 19 (3): 197-209. https://www.ncbi.nlm.nih.gov/pubmed/23348042
76. Iyengar NM, Hudis CA, Dannenberg AJ (2013) Obesity and inflammation: new insights into breast cancer development and progression. Am. Soc. Clin. Oncol. Educ. Book 33: 46-51. https://www.ncbi.nlm.nih.gov/pubmed/23714453.
77. Bracht JR, Vieira-Potter VJ, De Souza Santos R, Oz OK, Palmer BF, Clegg DJ (2020) The role of estrogens in the adipose tissue milieu. Ann. N. Y. Acad. Sci. 1461 (1): 127-143. https://www.ncbi.nlm.nih.gov/pubmed/31868931.
78. Esposito E, Cuzzocrea S (2010) Antiinflammatory activity of melatonin in central nervous system. Curr. Neuropharmacol. 8 (3): 228-242. https://www.ncbi.nlm.nih.gov/pubmed/21358973.
79. Pandi-Perumal SR, Trakht I, Srinivasan V, Spence DW, Maestroni GJ, Zisapel N, Cardinali DP (2008) Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 85 (3): 335-353. https://www.ncbi.nlm.nih.gov/pubmed/18571301.
80. Reppert SM, Weaver DR, Ebisawa T (1994) Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron 13 (5): 1177-1185. https://www.ncbi.nlm.nih.gov/pubmed/7946354.
81. Becker-Andre M, Wiesenberg I, Schaeren-Wiemers N, Andre E, Missbach M, Saurat JH, Carlberg C (1994) Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J. Biol. Chem. 269 (46): 28531-28534. https://www.ncbi.nlm.nih.gov/pubmed/7961794.
82. Benitez-King G, Huerto-Delgadillo L, & Anton-Tay F (1993) Binding of 3H-melatonin to calmodulin. Life Sci. 53 (3): 201-207. Https://www.ncbi.nlm.nih.gov/pubmed/8321083.
83. Ryu V, Zarebidaki E, Albers HE, Xue B, Bartness TJ (2018) Short photoperiod reverses obesity in Siberian hamsters via sympathetically induced lipolysis and browning in adipose tissue. Physiol. Behav. 190: 11-20. https://www.ncbi.nlm.nih.gov/pubmed/28694154.
84. González A, Alvarez-García V, Martínez-Campa C, Alonso-González C, Cos S (2012) Melatonin promotes differentiation of 3T3-L1 fibroblasts. J. Pineal Res. 52 (1): 12-20. https://www.ncbi.nlm.nih.gov/pubmed/21718362.
85. Kato H, Tanaka G, Masuda S, Ogasawara J, Sakurai T, Kizaki T, Ohno H, Izawa T (2015) Melatonin promotes adipogenesis and mitochondrial biogenesis in 3T3-L1 preadipocytes. J. Pineal Res. 59 (2): 267-275. https://www.ncbi.nlm.nih.gov/pubmed/26123001.
86. Yang W, Tang K, Wang Y, Zhang Y, Zan L (2017) Melatonin promotes triacylglycerol accumulation via MT2 receptor during differentiation in bovine intramuscular preadipocytes. Sci. Rep. 7 (1): 15080. https://www.ncbi.nlm.nih.gov/pubmed/29118419.
87. Oliveira AC, Andreotti S, Sertie RAL, Campana AB, de Proença ARG, Vasconcelos RP, Oliveira KA, Coelho-de-Souza AN, Donato-Junior J, Lima FB (2018) Combined treatment with melatonin and insulin improves glycemic control, white adipose tissue metabolism and reproductive axis of diabetic male rats. Life Sci. 199: 158-166. https://www.ncbi.nlm.nih.gov/pubmed/29501522.
88. Fernandez Vazquez G, Reiter RJ, Agil A (2018) Melatonin increases brown adipose tissue mass and function in Zucker diabetic fatty rats: implications for obesity control. J. Pineal. Res. 64 (4): e12472. https://www.ncbi.nlm.nih.gov/pubmed/29405372.
89. Agil A, Navarro-Alarcón M, Ruiz R, Abuhamadah S, El-Mir MY, Vázquez GF (2011) Beneficial effects of melatonin on obesity and lipid profile in young Zucker diabetic fatty rats. J. Pineal Res. 50 (2): 207-212. https://www.ncbi.nlm.nih.gov/pubmed/21087312.
90. Favero G, Stacchiotti A, Castrezzati S, Bonomini F, Albanese M, Rezzani R, Rodella LF (2015) Melatonin reduces obesity and restores adipokine patterns and metabolism in obese (ob/ob) mice. Nutrit. Res. 35 (10): 891-900. https://www.ncbi.nlm.nih.gov/pubmed/26250620.
91. Suriagandhi V, Nachiappan V (2022) Protective effects of melatonin against obesity-induced by leptin resistance. Behav. Brain Res. 417: 113598. https://www.ncbi.nlm.nih.gov/pubmed/34563600.
92. Szewczyk-Golec K, Woźniak A, Reiter RJ (2015) Inter-relationships of the chronobiotic, melatonin, with leptin and adiponectin: implications for obesity. J. Pineal Res. 59 (3): 277-291. https://www.ncbi.nlm.nih.gov/pubmed/26103557.
93. Agil A, Reiter RJ, Jiménez-Aranda A, Ibán-Arias R, Navarro-Alarcón M, Marchal JA, Adem A, Fernández-Vázquez G (2013) Melatonin ameliorates low-grade inflammation and oxidative stress in young Zucker diabetic fatty rats. J. Pineal Res. 54 (4): 381-388. https://www.ncbi.nlm.nih.gov/pubmed/23020082.
94. Yin J, Li Y, Han H, Chen S, Gao J, Liu G, Wu X, Deng J, Yu Q, Huang X, Fang R, Li T, Reiter RJ, Zhang D, Zhu C, Zhu G, Ren W, Yin Y (2018) Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. J. Pineal Res. 65 (4): e12524.
95. Wolden-Hanson T, Mitton DR, McCants RL, Yellon SM, Wilkinson CW, Matsumoto AM, Rasmussen DD (2000) Daily melatonin administration to middle-aged male rats suppresses body weight, intraabdominal adiposity, and plasma leptin and insulin independent of food intake and total body fat. Endocrinol. 141 (2): 487-497. https://www.ncbi.nlm.nih.gov/pubmed/30230594
96. Rios-Lugo MJ, Cano P, Jimenez-Ortega V, Fernandez-Mateos MP, Scacchi PA, Cardinali DP, Esquifino AI (2010) Melatonin effect on plasma adiponectin, leptin, insulin, glucose, triglycerides and cholesterol in normal and high fat-fed rats. J. Pineal Res. 49 (4): 342-348. https://www.ncbi.nlm.nih.gov/pubmed/20663045.
97. Terron MP, Delgado-Adamez J, Pariente JA, Barriga C, Paredes SD, Rodriguez AB (2013) Melatonin reduces body weight gain and increases nocturnal activity in male Wistar rats. Physiol. Behav. 118: 8-13. https://www.ncbi.nlm.nih.gov/pubmed/23643827.
98. Mustonen AM, Nieminen P, Hyvarinen H (2002) Effects of continuous light and melatonin treatment on energy metabolism of the rat. J. Endocrinol. Invest. 25 (8): 7 16-723. 723 https://www.ncbi.nlm.nih.gov/pubmed/12240904.
99. Alonso-Vale MI, Andreotti S, Peres SB, Anhe GF, das Neves Borges-Silva C, Neto JC, Lima FB (2005) Melatonin enhances leptin expression by rat adipocytes in the presence of insulin. Am. J. Physiol. Endocrinol. Metab. 288 (4): E805-812. https://www.ncbi.nlm.nih.gov/pubmed/15572654.
100. Rasmussen DD, Mitton DR, Larsen SA, Yellon SM (2001) Aging-dependent changes in the effect of daily melatonin supplementation on rat metabolic and behavioral responses. J. Pineal Res. 31 (1): 89-94. https://www.ncbi.nlm.nih.gov/pubmed/11485011.
101. Liu K, Yu W, Wei W, Zhang X, Tian Y, Sherif M, Liu X, Dong C, Wu W, Zhang L, Chen J (2019) Melatonin reduces intramuscular fat deposition by promoting lipolysis and increasing mitochondrial function. J. Lipid Res. 60 (4): 767-782. https://www.ncbi.nlm.nih.gov/pubmed/30552289.
102. Thorp AA & Schlaich MP (2015) Relevance of sympathetic nervous system activation in obesity and metabolic syndrome. J. Diabetes Res. 2015: 341583. https://www.ncbi.nlm.nih.gov/pubmed/26064978.
103. Demas GE, Bartness TJ (2001) Direct innervation of white fat and adrenal medullary catecholamines mediate photoperiodic changes in body fat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281 (5): R1499-1505. https://www.ncbi.nlm.nih.gov/pubmed/11641121.
104. Menéndez-Menéndez J, Martínez-Campa C (2018) Melatonin: An anti-tumor agent in hormone-dependent cancers. Inter. J. Endocrinol. 2018: 3271948. https://www.ncbi.nlm.nih.gov/pubmed/30386380.
105. Veiga ECA, Simões R, Valenti VE, Cipolla-Neto J, Abreu LC, Barros EPM, Sorpreso ICE, Baracat MCP, Baracat EC, Soares Junior JM (2019) Repercussions of melatonin on the risk of breast cancer: a systematic review and meta-analysis. Rev. Assoc. Med. Brasil. 65 (5): 699-705. https://www.ncbi.nlm.nih.gov/pubmed/31166448.
106. Chottanapund S, Van Duursen MB, Navasumrit P, Hunsonti P, Timtavorn S, Ruchirawat M, Van den Berg M (2014) Anti-aromatase effect of resveratrol and melatonin on hormonal positive breast cancer cells co-cultured with breast adipose fibroblasts. Toxicol. In Vitro 7: 1 215-1221. https://www.ncbi.nlm.nih.gov/pubmed/24929094.
107. Cipolla-Neto J, Amaral FG, Soares JM, Jr., Gallo CC, Furtado A, Cavaco JE, Gonçalves I, Santos CRA, Quintela T (2022) The Crosstalk between melatonin and sex steroid hormones. Neuroendocrinol. 112 (2): 1 15-129. https://www.ncbi.nlm.nih.gov/pubmed/33774638.
108. Otsuka F (2018) Interaction of melatonin and BMP-6 in ovarian steroidogenesis. Vit. Horm. 107: 137-153. https://www.ncbi.nlm.nih.gov/pubmed/29544628.
109. Guan Q, Wang Z, Cao J, Dong Y, Chen Y (2021) Mechanisms of melatonin in obesity: A review. Int. J. Mol. Sci. 23 (1): 218. https://www.ncbi.nlm.nih.gov/pubmed/35008644.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


