Physiological processes underpinning the ubiquitous benefits and interactions of melatonin, butyrate and green tea in neurodegenerative conditions
Nutraceuticals and melatonergic pathway
Abstract
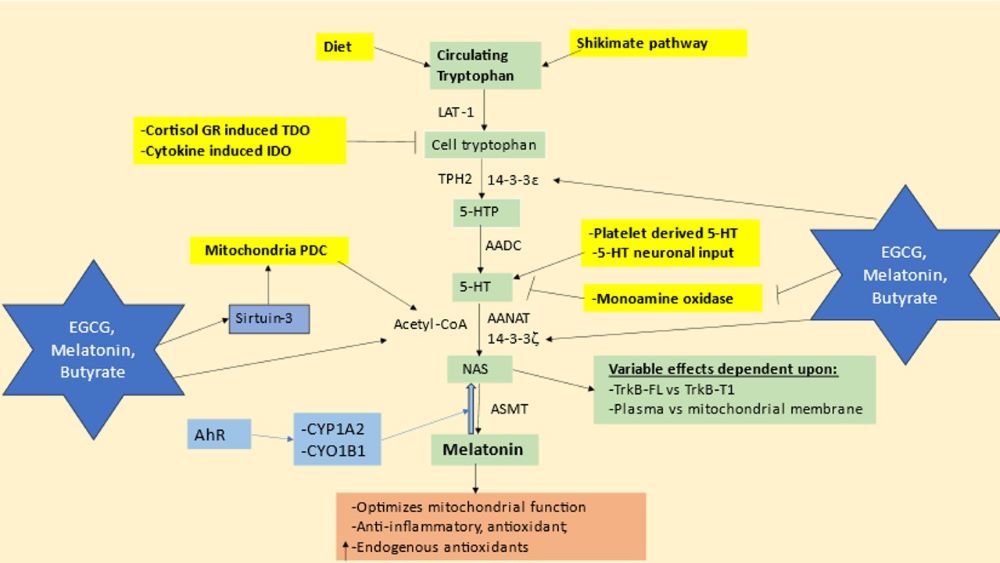
There is a growing dissatisfaction at the lack of progress in treating neurodegenerative conditions, such as Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis. No current pharmaceuticals have any significant impact on the pathophysiological changes occurring in such neurodegenerative conditions. More promising has been the utilization of nutraceuticals, a number of which show preventative and treatment benefits. This article reviews the beneficial effects of melatonin, sodium butyrate and epigallocatechin gallate (EGCG) in the management of the pathophysiological changes underpinning neurodegenerative conditions. It is proposed that all three nutraceuticals upregulate the tryptophan-melatonin pathway, which may be particularly important in astrocytes given astrocyte regulation of neuronal energy supply and antioxidants, including released melatonin. Alterations in the tryptophan-melatonin pathway are intimately intertwined with changes in the kynurenine pathway and its neuroregulatory products, including kynurenic acid and quinolinic acid. This article places these changes in the tryptophan-melatonin pathways within a novel circadian-systemic interaction, involving the regulation of the night-time rise in cortisol culminating in the morning cortisol awakening response that mediates effects via glucocorticoid receptor (GR) activation. The night-time and morning GR activation is suppressed by melatonin, gut microbiome derived butyrate and bcl2-associated athanogene (BAG)-1. As melatonin, butyrate and BAG-1 decrease over age, there is a heightened level of GR nuclear translocation with age at night and early morning. This is exemplified by the 10-fold decrease in pineal melatonin in people in their 9th, versus 2nd, decade of life. The ‘battle’ of melatonin/butyrate/EGCG versus cortisol/GR for influence on cellular function, microenvironment homeostasis and systemic system (immune) regulation at night and early morning shapes how the body and brain are prepared for the coming day and drives the emergence of aging associated neurodegenerative conditions. It is upon such processes that melatonin, butyrate and EGCG have their impacts.
References
2. Anderson G, Maes, M (2015) Pharmaceutical and nutritional benefits in alzheimer's disease via convergence on the melatoninergic pathways. Chap 2: FCDR-Alzheimer Disorder, Vol. 4, 50-127.
3. Shukla M, Vincent B (2023) Melatonin as a harmonizing factor of circadian rhythms, neuronal cell cycle and neurogenesis: Additional arguments for its therapeutic use in Alzheimer's disease. Curr. Neuropharmacol. 21 (5): 1273-1298. doi: 10.2174/1570159X21666230314142505.
4. Anderson G (2023) A more holistic perspective of Alzheimer’s disease: Roles of gut microbiome, adipocytes, HPA axis, melatonergic pathway and astrocyte mitochondria in the emergence of autoimmunity. Front. Biosc. (Landmark). 28 (12): 355. doi: 10.31083/j.fbl2812355.
5. Wang C, Zheng D, Weng F, Jin Y, He L (2022) Sodium butyrate ameliorates the cognitive impairment of Alzheimer's disease by regulating the metabolism of astrocytes. Psychopharmacology (Berl). 239 (1): 215-227. doi: 10.1007/s00213-021-06025-0.
6. Valverde-Salazar V, Ruiz-Gabarre D, García-Escudero V (2023) Alzheimer's disease and green tea: Epigallocatechin-3-gallate as a modulator of inflammation and oxidative stress. Antioxidants (Basel). 12 (7), 1460. doi: 10.3390/antiox12071460.
7. Tchekalarova J, Tzoneva R (2023) Oxidative stress and aging as risk factors for Alzheimer's disease and parkinson's disease: The role of the antioxidant melatonin. Int. J. Mol. Sci. 24 (3): 3022. doi: 10.3390/ijms24033022.
8. Gao C, Li B, He Y, Huang P, Du J, He G, et al (2023) Early changes of fecal short-chain fatty acid levels in patients with mild cognitive impairments. CNS. Neurosci. Ther. 29 (11): 3657-3666. doi: 10.1111/cns.14252.
9. Rani A, Saini V, Patra P, Prashar T, Pandey RK, Mishra A, et al. (2023) Epigallocatechin gallate: A multifaceted molecule for neurological disorders and neurotropic viral infections. ACS. Chem. Neurosci. 14 (17): 2968-2980. doi: 10.1021/acschemneuro.3c00368.
10. Iftikhar S, Sameer HM, Zainab (2023) Significant potential of melatonin therapy in Parkinson's disease - a meta-analysis of randomized controlled trials. Front. Neurol. 14: 1265789. doi: 10.3389/fneur.2023.1265789.
11. Anderson G, Rodriguez M, Reiter RJ (2019) Multiple sclerosis: Melatonin, orexin, and ceramide interact with platelet activation coagulation factors and gut-microbiome-derived butyrate in the circadian dysregulation of mitochondria in glia and immune cells. Int. J. Mol. Sci. 20 (21): 5500. doi: 10.3390/ijms20215500.
12. Ormstad H, Simonsen CS, Broch L, Maes DM, Anderson G, Celius EG (2020) Chronic fatigue and depression due to multiple sclerosis: Immune-inflammatory pathways, tryptophan catabolites and the gut-brain axis as possible shared pathways. Mult. Scler. Relat. Disord. 46: 102533. doi: 10.1016/j.msard.2020.102533.
13. Moon E, Kim K, Partonen T, Linnaranta O (2022) Role of melatonin in the management of sleep and circadian disorders in the context of psychiatric illness. Curr. Psychiatry. Rep. 24 (11): 623-634. doi: 10.1007/s11920-022-01369-6.
14. Sublette ME, Cheung S, Lieberman E, Hu S, Mann JJ, Uhlemann AC, Miller JM (2021) Bipolar disorder and the gut microbiome: A systematic review. Bipolar. Disord. 23 (6): 544-564. doi: 10.1111/bdi.13049.
15. Rothenberg DO, Zhang L (2019) Mechanisms underlying the anti-depressive effects of regular tea consumption. Nutrients 11 (6): 1361. doi: 10.3390/nu11061361.
16. Rodríguez-Santana C, López-Rodríguez A, Martinez-Ruiz L, Florido J, Cela O, Capitanio N, et al (2023) The Relationship between clock genes, sirtuin 1, and mitochondrial activity in head and neck squamous cell cancer: Effects of melatonin treatment. Int. J. Mol. Sci. 24 (19): 15030. doi: 10.3390/ijms241915030.
17. Jaye K, Alsherbiny MA, Chang D, Li CG, Bhuyan DJ (2023) Mechanistic insights into the anti-proliferative action of gut microbial metabolites against breast adenocarcinoma cells. Int. J. Mol. Sci. 24 (20): 15053. doi: 10.3390/ijms242015053.
18. Wang C, Bai M, Sun Z, Yao N, Zhang A, Guo S, et al. (2023) Epigallocatechin-3-gallate and cancer: focus on the role of microRNAs. Cancer Cell. Int. 23 (1): 241. doi: 10.1186/s12935-023-03081-8.
19. Wei XY, Zeng YF, Guo QH, Liu JJ, Yin N, Liu Y, et al. (2023) Cardioprotective effect of epigallocatechin gallate in myocardial ischemia/reperfusion injury and myocardial infarction: a meta-analysis in preclinical animal studies. Sci. Rep. 13 (1): 14050. doi: 10.1038/s41598-023-41275-2.
20. Modrego J, Ortega-Hernández A, Goirigolzarri J, Restrepo-Córdoba MA, Bäuerl C, Cortés-Macías E, et al (2023) Gut microbiota and derived short-chain fatty acids are linked to evolution of heart failure patients. Int. J. Mol. Sci. 24 (18): 13892. doi: 10.3390/ijms241813892.
21. Asor E, Ben-Shachar D (2012) Platelets: A possible glance into brain biological processes in schizophrenia. World. J. Psychiatry 2 (6): 124-133.
22. Anderson G (2019) Daytime orexin and night-time melatonin regulation of mitochondria melatonin:roles in circadian oscillations systemically and centrally in breast cancer symptomatology. Melatonin. Res. 2 (4): 1-8. doi: org/10.32794/mr11250037.
23. Reiter RJ, Sharma R, Ma Q, Rosales-Corral SA, Acuna-Castroviejo D, Escames G (2019) Inhibition of mitochondrial pyruvate dehydrogenase kinase: A proposed mechanism by which melatonin causes cancer cells to overcome cytosolic glycolysis, reduce tumor biomass and reverse insensitivity to chemotherapy. Melatonin Res. 2 (3): 105-119. doi:10.32794/mr11250033.
24. Anderson G (2023) Why are aging and stress associated with dementia, cancer, and other diverse medical conditions? Role of pineal melatonin interactions with the HPA axis in mitochondrial regulation via BAG-1. Melatonin Res. 6 (3), 345-371. doi: org/10.32794/mr112500158.
25. Shen S, Liao Q, Wong YK, Chen X, Yang C, Xu C, et al. (2022) The role of melatonin in the treatment of type 2 diabetes mellitus and Alzheimer's disease. Int. J. Biol. Sci. 18 (3): 983-994. doi: 10.7150/ijbs.66871.
26. de Melo LGP, Nunes SOV, Anderson G, Vargas HO, Barbosa DS, Galecki P, et al. (2017) Shared metabolic and immune-inflammatory, oxidative and nitrosative stress pathways in the metabolic syndrome and mood disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry. 78: 34-50. doi: 10.1016/j.pnpbp.2017.04.027.
27. Asefy Z, Khusro A, Mammadova S, Hoseinnejhad S, Eftekhari A, Alghamdi S, et al. (2021) Melatonin hormone as a therapeutic weapon against neurodegenerative diseases. Cell. Mol. Biol. (Noisy-le-grand). 67 (3): 99-106. doi: 10.14715/cmb/2021.67.3.13.
28. Wang H, Pu Y, Luo L, Li Y, Zhang Y, Cao Z (2018) Membrane receptor-independent inhibitory effect of melatonin on androgen production in porcine theca cells. Theriogenology 118: 63-71. doi: 10.1016/j.theriogenology.2018.05.042.
29. Liu YJ, Zhuang J, Zhu HY, Shen YX, Tan ZL, Zhou JN (2007) Cultured rat cortical astrocytes synthesize melatonin: absence of a diurnal rhythm. J. Pineal. Res. 43 (3): 232-8. doi: 10.1111/j.1600-079X.2007.00466.x.
30. Markus RP, Fernandes PA, Kinker GS, da Silveira Cruz-Machado S, Marçola M (2018) Immune-pineal axis – acute inflammatory responses coordinate melatonin synthesis by pinealocytes and phagocytes. Br. J. Pharmacol. 175: 3239–3250.
31. Muxel SM, Pires-Lapa MA, Monteiro AW, Cecon E, Tamura EK, Floeter-Winter LM, et al. (2012) NF-κB drives the synthesis of melatonin in RAW 264.7 macrophages by inducing the transcription of the arylalkylamine-N-acetyltransferase (AA-NAT) gene. PLoS One 7 (12): e52010. doi: 10.1371/journal.pone.0052010.
32. Fu S, Kuwahara M, Uchida Y, Koudo S, Hayashi D, Shimomura Y, et al. (2019) Circadian production of melatonin in cartilage modifies rhythmic gene expression. J. Endocrinol. JOE-19-0022: R2. doi: 10.1530/JOE-19-0022.
33. Anderson G, Maes M (2020) Gut dysbiosis dysregulates central and systemic homeostasis via suboptimal mitochondrial function: assessment, treatment and classification implications. Curr. Top. Med. Chem. 20 (7): 524-539. doi: 10.2174/1568026620666200131094445.
34. Ernesto JT, Damasio CM, Gontijo VS, Gasparotto J, Viegas C Jr (2023) The role of microbiota-gut-brain axis in neurodegenerative diseases: biochemical and therapeutic aspects. Explor. Neuroprot. Ther. 3: 71–89. doi.org/10.37349/ent.2023.00038.
35. Liu L, Cao Q, Gao W, Li B, Xia Z, Zhao B (2021) Melatonin protects against focal cerebral ischemia-reperfusion injury in diabetic mice by ameliorating mitochondrial impairments: involvement of the Akt-SIRT3-SOD2 signaling pathway. Aging (Albany NY). 13 (12): 16105-16123. doi: 10.18632/aging.203137.
36. Chen M, Hui S, Lang H, Zhou M, Zhang Y, Kang C, et al. (2019) SIRT3 deficiency promotes high-fat diet-induced nonalcoholic fatty liver disease in correlation with impaired intestinal permeability through gut microbial dysbiosis. Mol. Nutr. Food. Res. 63 (4): e1800612. doi: 10.1002/mnfr.201800612.
37. Wei T, Huang G, Liu P, Gao J, Huang C, Sun M, et al. (2019) Sirtuin 3-mediated pyruvate dehydrogenase activity determines brown adipocytes phenotype under high-salt conditions. Cell. Death Dis. 10 (8): 614. doi: 10.1038/s41419-019-1834-4.
38. Ozden O, Park SH, Wagner BA, Song HY, Zhu Y, Vassilopoulos A, et al. (2014) SIRT3 deacetylates and increases pyruvate dehydrogenase activity in cancer cells. Free. Radic. Biol. Med. 76: 163-172. doi: 10.1016/j.freeradbiomed.2014.08.001.
39. Cortés-Rojo C, Vargas-Vargas MA, Olmos-Orizaba BE, Rodríguez-Orozco AR, Calderón-Cortés E (2020) Interplay between NADH oxidation by complex I, glutathione redox state and sirtuin-3, and its role in the development of insulin resistance. Biochim. Biophys. Acta Mol. Basis. Dis. 1866 (8): 165801. doi: 10.1016/j.bbadis.2020.165801.
40. He J, Jiang BH (2016) Interplay between reactive oxygen species and microRNAs in cancer. Curr. Pharmacol. Rep. 2 (2): 82-90. doi: 10.1007/s40495-016-0051-4.
41. Garcia G, Pinto S, Ferreira S, Lopes D, Serrador MJ, Fernandes A, et al. (2022) Emerging role of miR-21-5p in neuron-glia dysregulation and exosome transfer using multiple models of Alzheimer's disease. Cells 11 (21): 3377. doi: 10.3390/cells11213377.
42. Anderson G, Reiter RJ (2020) Melatonin: Roles in influenza, Covid-19, and other viral infections. Rev. Med. Virol. 30 (3): e2109. doi: 10.1002/rmv.2109.
43. Storozhuk M, Lee S, Lee JI, Park J (2023) Green tea consumption and the COVID-19 omicron pandemic era: Pharmacology and epidemiology. Life (Basel). 13 (3): 852. doi: 10.3390/life13030852.
44. Kim HS, Quon MJ, Kim JA (2014) New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox. Biol. 2: 187-95. doi: 10.1016/j.redox.2013.12.022.
45. Sarkar J, Das M, Howlader MSI, Prateeksha P, Barthels D, Das H (2022) Epigallocatechin-3-gallate inhibits osteoclastic differentiation by modulating mitophagy and mitochondrial functions. Cell Death Dis. 13 (10): 908. doi: 10.1038/s41419-022-05343-1.
46. Hong H, Li Y, Su B (2023) Identification of circulating miR-125b as a potential biomarker of Alzheimer's disease in APP/PS1 transgenic mouse. J. Alzheimers Dis. 59 (4): 1449-1458. doi: 10.3233/JAD-170156.
47. Shirazi-Tehrani E, Chamasemani A, Firouzabadi N, Mousaei M (2022) ncRNAs and polyphenols: New therapeutic strategies for hypertension. RNA. Biol. 19 (1): 575-587. doi: 10.1080/15476286.2022.2066335.
48. Liu C, Liu J, Wang W, Yang M, Chi K, Xu Y, et al. (2023) Epigallocatechin gallate alleviates staphylococcal enterotoxin a-induced intestinal barrier damage by regulating gut microbiota and inhibiting the TLR4-NF-κB/MAPKs-NLRP3 inflammatory cascade. J. Agric. Food Chem. 71 (43): 16286-16302. doi: 10.1021/acs.jafc.3c04526.
49. Hsieh MH, Cui ZY, Yang AL, Nhu NT, Ting SY, Yu SH, et al. (2021) Cerebral cortex apoptosis in early aged hypertension: Effects of epigallocatechin-3-gallate. Front. Aging Neurosci. 13: 705304. doi: 10.3389/fnagi.2021.705304.
50. Lin SM, Wang SW, Ho SC, Tang YL (2010) Protective effect of green tea (-)-epigallocatechin-3-gallate against the monoamine oxidase B enzyme activity increase in adult rat brains. Nutrition 26 (11-12): 1195-200. doi: 10.1016/j.nut.2009.11.022.
51. Sharma R, Kumar R, Sharma A, Goel A, Padwad Y (2022) Long-term consumption of green tea EGCG enhances murine health span by mitigating multiple aspects of cellular senescence in mitotic and post-mitotic tissues, gut dysbiosis, and immunosenescence. J. Nutr. Biochem. 107: 109068. doi: 10.1016/j.jnutbio.2022.109068.
52. Tan DX, Manchester LC, Liu X, Rosales-Corral SA, Acuna-Castroviejo D, Reiter RJ (2013) Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin's primary function and evolution in eukaryotes. J. Pineal. Res. 54 (2): 127-38. doi: 10.1111/jpi.12026.
53. Winge I, McKinney JA, Ying M, D’Santos CS, Kleppe R, Knappskog PM, et al. (2008) Activation and stabilization of human tryptophan hydroxylase 2 by phosphorylation and 14-3-3 binding. Biochem. J. 410: 195–204.
54. Ganguly S, Weller JL, Ho A, Chemineau P, Malpaux B, Klein DC (2005) Melatonin synthesis: 14-3-3-dependent activation and inhibition of arylalkylamine N-acetyltransferase mediated by phosphoserine-205. Proc. Natl. Acad. Sci. USA. 102 (4): 1222-7. doi: 10.1073/pnas.0406871102.
55. Abudahab S, Price ET, Dozmorov MG, Deshpande LS, McClay JL (2023) The aryl hydrocarbon receptor, epigenetics and the aging process. J. Nutr. Health Aging 27 (4): 291-300. doi: 10.1007/s12603-023-1908-1.
56. Salminen A. Aryl hydrocarbon receptor (AhR) impairs circadian regulation: Impact on the aging process (2023) Ageing Res. Rev. 87: 101928. doi: 10.1016/j.arr.2023.101928.
57. Ramos-García NA, Orozco-Ibarra M, Estudillo E, Elizondo G, Gómez Apo E, Chávez Macías LG, et al. (2020) Aryl hydrocarbon receptor in post-mortem hippocampus and in serum from young, elder, and Alzheimer's patients. Int. J. Mol. Sci. 21 (6): 1983. doi: 10.3390/ijms21061983.
58. Zhou Y, Zhao WJ, Quan W, Qiao CM, Cui C, Hong H, et al. (2021) Dynamic changes of activated AHR in microglia and astrocytes in the substantia nigra-striatum system in an MPTP-induced Parkinson's disease mouse model. Brain Res. Bull. 176: 174-183. doi: 10.1016/j.brainresbull.2021.08.013.
59. Chatterjee P, Banerjee S (2023) Unveiling the mechanistic role of the Aryl hydrocarbon receptor in environmentally induced Breast cancer. Biochem. Pharmacol. 218: 115866. doi: 10.1016/j.bcp.2023.115866.
60. Mokkawes T, De Visser T, Cao Y, De Visser SP (2023) Melatonin activation by human cytochrome P450 enzymes: A comparison between different isozymes. Molecules 28 (19): 6961. doi: 10.3390/molecules28196961.
61. Ma X, Idle JR, Krausz KW, Gonzalez FJ (2005) Metabolism of melatonin by human cytochromes p450. Drug Metab. Dispos. 33 (4): 489-94. doi: 10.1124/dmd.104.002410.
62. Anderson G (2020) Glioblastoma chemoresistance: roles of the mitochondrial melatonergic pathway. Cancer Drug Resist. 3 (3): 334-355. doi: 10.20517/cdr.2020.17.
63. Yoo DY, Nam SM, Kim W, Lee CH, Won MH, Hwang IK, et al. (2011) N-acetylserotonin increases cell proliferation and differentiating neuroblasts with tertiary dendrites through upregulation of brain-derived neurotrophic factor in the mouse dentate gyrus. J. Vet. Med. Sci. 73 (11): 1411-6. doi: 10.1292/jvms.11-0123.
64. Jang SW, Liu X, Pradoldej S, Tosini G, Chang Q, Iuvone PM, et al. (2010) N-acetylserotonin activates TrkB receptor in a circadian rhythm. Proc. Natl. Acad. Sci. USA. 107 (8): 3876-81. doi: 10.1073/pnas.0912531107.
65. Tessarollo L, Yanpallewar S (2022) TrkB Truncated isoform receptors as transducers and determinants of BDNF functions. Front. Neurosci. 16: 847572. doi: 10.3389/fnins.2022.847572.
66. Anderson G (2022) Amyotrophic lateral sclerosis pathoetiology and pathophysiology: roles of astrocytes, gut microbiome, and muscle interactions via the mitochondrial melatonergic pathway, with disruption by glyphosate-based herbicides. Int. J. Mol. Sci. 24 (1): 587. doi: 10.3390/ijms24010587.
67. Modoux M, Rolhion N, Lefevre JH, Oeuvray C, Nádvorník P, Illes P, et al. (2022) Butyrate acts through HDAC inhibition to enhance aryl hydrocarbon receptor activation by gut microbiota-derived ligands. Gut Microbes 14 (1): 2105637. doi: 10.1080/19490976.2022.2105637.
68. Wu J, Jiang Z, Zhang H, Liang W, Huang W, Zhang H, et al. (2018) Sodium butyrate attenuates diabetes-induced aortic endothelial dysfunction via P300-mediated transcriptional activation of Nrf2. Free. Radic. Biol. Med. 124: 454-465. doi: 10.1016/j.freeradbiomed.2018.06.034.
69. Anderson G, Carbone A, Mazzoccoli G (2021) Tryptophan metabolites and aryl hydrocarbon receptor in severe acute respiratory syndrome, coronavirus-2 (SARS-CoV-2) pathophysiology. Int. J. Mol. Sci. 22 (4): 1597. doi: 10.3390/ijms22041597.
70. Ayyadurai VAS, Deonikar P (2023) Attenuation of aging-related oxidative stress pathways by phytonutrients: a computational systems biology analysis. Nutrients 15 (17): 3762. doi: 10.3390/nu15173762.
71. Govindarajulu M, Ramesh S, Neel L, Fabbrini M, Buabeid M, Fujihashi A, et al. (2021) Nutraceutical based SIRT3 activators as therapeutic targets in Alzheimer's disease. Neurochem. Int. 144: 104958. doi: 10.1016/j.neuint.2021.104958.
72. Jin CJ, Engstler AJ, Sellmann C, Ziegenhardt D, Landmann M, Kanuri G, et al. (2016) Sodium butyrate protects mice from the development of the early signs of non-alcoholic fatty liver disease: role of melatonin and lipid peroxidation. Br. J. Nutr. 116 (10): 1682-1693. doi: 10.1017/S0007114516004025.
73. Swanson GR, Gorenz A, Shaikh M, Desai V, Forsyth C, Fogg L, et al. (2015) Decreased melatonin secretion is associated with increased intestinal permeability and marker of endotoxemia in alcoholics. Am. J. Physiol. Gastrointest. Liver Physiol. 308 (12): G1004-11. doi: 10.1152/ajpgi.00002.2015.
74. Wei R, Liu X, Wang Y, Dong J, Wu F, Mackenzie GG, Su Z (2021) (-)-Epigallocatechin-3-gallate mitigates cyclophosphamide-induced intestinal injury by modulating the tight junctions, inflammation and dysbiosis in mice. Food Funct. 12 (22): 11671-11685. doi: 10.1039/d1fo01848e.
75. Németh H, Toldi J, Vécsei L (2005) Role of kynurenines in the central and peripheral nervous systems. Curr. Neurovasc. Res. 2 (3): 249-60. doi: 10.2174/1567202054368326.
76. Cho E, Park J, Hwang EM, Kim HW, Park JY (2023) 14-3-3γ haploinsufficiency leads to altered dopamine pathway and Parkinson's disease-like motor incoordination in mice. Mol. Brain 16 (1): 2. doi: 10.1186/s13041-022-00990-z.
77. Cho E, Park JY (2020) Emerging roles of 14-3-3γ in the brain disorder. BMB. Rep. 53 (10), 500-511. doi: 10.5483/BMBRep.2020.53.10.158.
78. Kim AR, Kim KM, Byun MR, Hwang JH, Park JI, Oh HT, et al. (2017) (-)-Epigallocatechin-3-gallate stimulates myogenic differentiation through TAZ activation. Biochem. Biophys. Res. Commun. 486 (2): 378-384. doi: 10.1016/j.bbrc.2017.03.049.
79. Weinreb O, Amit T, Youdim MB (2007) A novel approach of proteomics and transcriptomics to study the mechanism of action of the antioxidant-iron chelator green tea polyphenol (-)-epigallocatechin-3-gallate. Free. Radic. Biol. Med. 43 (4): 546-56. doi: 10.1016/j.freeradbiomed.2007.05.011.
80. Agosto LM, Mallory MJ, Ferretti MB, Blake D, Krick KS, Gazzara MR, et al. (2023) Alternative splicing of HDAC7 regulates its interaction with 14-3-3 proteins to alter histone marks and target gene expression. Cell Rep. 42 (3): 112273. doi: 10.1016/j.celrep.2023.112273.
81. Koh PO (2008) Melatonin attenuates the focal cerebral ischemic injury by inhibiting the dissociation of pBad from 14-3-3. J. Pineal Res. 44 (1): 101-6. doi: 10.1111/j.1600-079X.2007.00495.x.
82. Pagan C, Goubran-Botros H, Delorme R, Benabou M, Lemière N, Murray K, et al. (2017) Disruption of melatonin synthesis is associated with impaired 14-3-3 and miR-451 levels in patients with autism spectrum disorders. Sci. Rep. 7 (1): 2096. doi: 10.1038/s41598-017-02152-x.
83. Basri R, Fatima S, Jalil S, Imran A, Fatima N, Syed A, et al. (2023) 2-Oxoquinoline-based-thiosemicarbazones as multitargeting neurotherapeutics against Alzheimer's disease: In vitro and in silico studies of MAO and ChE inhibitors. Arch. Pharm. (Weinheim). 356 (11): e2300430. doi: 10.1002/ardp.202300430.
84. Manoharan A, Oh JM, Benny F, Kumar S, Abdelgawad MA, Ghoneim MM, et al. (2023) Assembling a cinnamyl pharmacophore in the C3-position of substituted isatins via microwave-assisted synthesis: Development of a new class of monoamine oxidase-B inhibitors for the treatment of Parkinson's disease. Molecules 28 (16): 6167. doi: 10.3390/molecules28166167.
85. Albertini C, Salerno A, Atzeni S, Uliassi E, Massenzio F, Maruca A, et al. (2022) Riluzole-rasagiline hybrids: Toward the development of multi-target-directed ligands for amyotrophic lateral sclerosis. ACS. Chem. Neurosci. 13 (15): 2252-2260. doi: 10.1021/acschemneuro.2c00261.
86. Mazzio EA, Harris N, Soliman KF (1998) Food constituents attenuate monoamine oxidase activity and peroxide levels in C6 astrocyte cells. Planta. Med. 64 (7): 603-6. doi: 10.1055/s-2006-957530.
87. Srivastav S, Neupane S, Bhurtel S, Katila N, Maharjan S, Choi H, et al. (2019) Probiotics mixture increases butyrate, and subsequently rescues the nigral dopaminergic neurons from MPTP and rotenone-induced neurotoxicity. J. Nutr. Biochem. 69: 73-86. doi: 10.1016/j.jnutbio.2019.03.021.
88. Elkamhawy A, Woo J, Gouda NA, Kim J, Nada H, Roh EJ, et al. (2021) Melatonin analogues potently inhibit MAO-B and protect PC12 cells against oxidative stress. Antioxidants (Basel). 10 (10): 1604. doi: 10.3390/antiox10101604.
89. Anderson G, Maes M (2014) Reconceptualizing adult neurogenesis: Role for sphingosine-1-phosphate and fibroblast growth factor-1 in co-ordinating astrocyte-neuronal precursor interactions. CNS. Neurol. Disord. Drug Targets 13 (1): 126-36. doi: 10.2174/18715273113126660132.
90. NaveenKumar SK, Hemshekhar M, Kemparaju K, Girish KS (2019) Hemin-induced platelet activation and ferroptosis is mediated through ROS-driven proteasomal activity and inflammasome activation: Protection by melatonin. Biochim. Biophys. Acta. Mol. Basis. Dis. 1865 (9): 2303-2316. doi: 10.1016/j.bbadis.2019.05.009.
91. Iida Y, Doi T, Matsushima-Nishiwaki R, Tokuda H, Ogura S, Kozawa O, et al. (2014) (-)-Epigallocatechin gallate selectively inhibits adenosine diphosphate stimulated human platelet activation: suppression of heat shock protein 27 phosphorylation via p38 mitogen activated protein kinase. Mol. Med. Rep. 10 (3): 1383-8. doi: 10.3892/mmr.2014.2389.
92. Bambakidis T, Dekker SE, Halaweish I, Liu B, Nikolian VC, Georgoff PE, et al. (2017) Valproic acid modulates platelet and coagulation function ex vivo. Blood. Coagul. Fibrinolysis 28 (6): 479-484. doi: 10.1097/MBC.0000000000000626.
93. Han SG, Han SS, Toborek M, Hennig B (2012) EGCG protects endothelial cells against PCB 126-induced inflammation through inhibition of AhR and induction of Nrf2-regulated genes. Toxicol. Appl. Pharmacol. 261 (2): 181-8. doi: 10.1016/j.taap.2012.03.024.
94. Chang TK, Chen J, Yang G, Yeung EY (2010) Inhibition of procarcinogen-bioactivating human CYP1A1, CYP1A2 and CYP1B1 enzymes by melatonin. J. Pineal Res. 48 (1): 55-64.
95. Slominski AT, Kim TK, Slominski RM, Song Y, Qayyum S, Placha W, et al. (2023) Melatonin and its metabolites can serve as agonists on the aryl hydrocarbon receptor and peroxisome proliferator-activated receptor gamma. Int. J. Mol. Sci. 24 (20): 15496. doi: 10.3390/ijms242015496.
96. Dopkins N, Becker W, Miranda K, Walla M, Nagarkatti P, Nagarkatti M (2021) Tryptamine attenuates experimental multiple sclerosis through activation of aryl hydrocarbon receptor. Front. Pharmacol. 11: 619265. doi: 10.3389/fphar.2020.619265.
97. White S, Mauer R, Lange C, Klimecki O, Huijbers W, Wirth M; Alzheimer's Disease Neuroimaging Initiative (2023) The effect of plasma cortisol on hippocampal atrophy and clinical progression in mild cognitive impairment. Alzheimers Dement. (Amst). 15 (3): e12463. doi: 10.1002/dad2.12463.
98. Bougea A, Stefanis L, Chrousos G (2022) Stress system and related biomarkers in Parkinson's disease. Adv. Clin. Chem. 111: 177-215.
99. Monachelli GG, Meyer M, Rodríguez G, Garay L, Sica RE, De Nicola AF, et al. (2011) Progesterone and cortisol levels in sporadic amyotrophic lateral sclerosis (sALS): Correlation with prognostic factors. Horm. Mol. Biol. Clin. Investig. 6 (1): 167-73. doi: 10.1515/HMBCI.2011.006.
100. Anderson G (2023) Melatonin, BAG-1 and cortisol circadian interactions in tumor pathogenesis. Roles of glucocorticoid receptor translocation site and mitochondrial melatonergic pathway. Exploration of targeted anti-tumor therapy. 4: 962–993. doi: 10.37349/etat.2023.00176.
101. Seckl J (2024) 11β-Hydroxysteroid dehydrogenase and the brain: Not (yet) lost in translation. J. Intern. Med. 295 (1): 20-37. doi: 10.1111/joim.13741.
102. Karasek M, Reiter RJ (2002) Melatonin and aging. Neuro. Endocrinol. Lett. 23 (Suppl 1): 14-16.
103. Patel R, Parmar N, Pramanik Palit S, Rathwa N, Ramachandran AV, Begum R (2022) Diabetes mellitus and melatonin: Where are we? Biochimie 202: 2-14. doi: 10.1016/j.biochi.2022.01.001.
104. Shukla PK, Meena AS, Pierre JF, Rao R (2022) Central role of intestinal epithelial glucocorticoid receptor in alcohol- and corticosterone-induced gut permeability and systemic response. FASEB J. 36 (1): e22061. doi: 10.1096/fj.202101424R.
105. Anderson G, Jacob A, Bellivier F, Geoffroy PA (2016) Bipolar disorder: The role of the kynurenine and melatonergic pathways. Curr. Pharm. Des. 22 (8): 987-1012. doi: 10.2174/1381612822666151214105314.
106. Law R, Clow A (2020) Stress, the cortisol awakening response and cognitive function. Int. Rev. Neurobiol. 150: 187-217. doi: 10.1016/bs.irn.2020.01.001.
107. Anderson G (2023) Why do anti-amyloid beta antibodies not work? Time to reconceptualize dementia pathophysiology by incorporating astrocyte melatonergic pathway desynchronization from amyloid-beta production. Braz. J. Psychiatry 45 (2): 89-92. doi: 10.47626/1516-4446-2022-2949.
108. Bernard M, Voisin P (2008) Photoreceptor-specific expression, light-dependent localization, and transcriptional targets of the zinc-finger protein Yin Yang 1 in the chicken retina. J. Neurochem. 105 (3): 595-604. doi: 10.1111/j.1471-4159.2007.05150.x.
109. Anderson G (2022) Depression pathophysiology: Astrocyte mitochondrial melatonergic pathway as crucial hub. Int. J. Mol. Sci. 24 (1): 350. doi: 10.3390/ijms24010350.
110. Zhai K, Huang Z, Huang Q, Tao W, Fang X, Zhang A, et al. (2021) Pharmacological inhibition of BACE1 suppresses glioblastoma growth by stimulating macrophage phagocytosis of tumor cells. Nat. Cancer 2 (11): 1136-1151. doi: 10.1038/s43018-021-00267-9.
111. Zayas-Santiago A, Martínez-Montemayor MM, Colón-Vázquez J, Ortiz-Soto G, Cirino-Simonet JG, Inyushin M (2022) Accumulation of amyloid beta (Aβ) and amyloid precursor protein (APP) in tumors formed by a mouse xenograft model of inflammatory breast cancer. FEBS Open Bio. 12 (1): 95-105. doi: 10.1002/2211-5463.13308.
112. Rannikko EH, Weber SS, Kahle PJ (2015) Exogenous α-synuclein induces toll-like receptor 4 dependent inflammatory responses in astrocytes. BMC. Neurosci. 16: 57. doi: 10.1186/s12868-015-0192-0.
113. Zhu K, Zhang Y, Zhang J, Zhou F, Zhang L, Wang S, et al (2020) Acetylation of Hsp90 reverses dexamethasone-mediated inhibition of insulin secretion. Toxicol. Lett. 320: 19-27. doi: 10.1016/j.toxlet.2019.11.022.
114. Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, et al. (2015) Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 17 (5): 681-689. doi: 10.1016/j.chom.2015.03.006.
115. Sakthivel SJ, Hay P, Touyz S, Currow D, Mannan H (2023) Association of participants who screened positive for night eating syndrome with physical health, sleep problems, and weight status in an Australian adult population. Eat Weight Disord. 28 (1): 77. doi: 10.1007/s40519-023-01603-x.
116. Anderson G (2023) Role of the night-time systemic processes and the astrocyte tryptophan-melatonin pathway in the regulation of α-synuclein and wider Parkinson’s disease pathophysiology. Preprints 2023: 2023110301. https://doi.org/10.20944/preprints202311.0301.v1.
117. Anderson G. (2022). Tumor microenvironment and metabolism: Role of the mitochondrial melatonergic pathway in determining intercellular interactions in a new dynamic homeostasis. Int. J. Mol. Sc. 24 (1): 311. https://doi.org/10.3390/ijms24010311.
118. Anderson G, Almulla AF, Reiter RJ, Maes M (2023) Redefining autoimmune disorders' pathoetiology: Implications for mood and psychotic disorders' association with neurodegenerative and classical autoimmune disorders. Cells 12 (9): 1237. https://doi.org/10.3390/cells12091237.
119. Rossi SP, Matzkin ME, Riviere E, Martinez G, Ponzio R, Levalle O, et al. (2023) Melatonin improves oxidative state and lactate metabolism in rodent Sertoli cells. Mol. Cell. Endocrinol. 576: 112034. doi: 10.1016/j.mce.2023.112034.
120. Hintzpeter J, Stapelfeld C, Loerz C, Martin HJ, Maser E (2014) Green tea and one of its constituents, epigallocatechine-3-gallate, are potent inhibitors of human 11β-hydroxysteroid dehydrogenase type 1. PLoS One 9 (1): be84468.
121. Sánchez-Vázquez FJ, López-Olmeda JF, Vera LM, Migaud H, López-Patiño MA, Míguez JM (2019) Environmental cycles, melatonin, and circadian control of stress response in fish. Front. Endocrinol. (Lausanne). 10: 279. doi: 10.3389/fendo.2019.00279.
122. Touitou Y, Bogdan A, Auzéby A, Touitou C (1989) Activity of melatonin and other pineal indoles on the in vitro synthesis of cortisol, cortisone, and adrenal androgens. J. Pineal Res. 6 (4): 341-350. doi: 10.1111/j.1600-079x.1989.tb00430.x.
123. Zhou J, Zhang J, Luo X, Li M, Yue Y, Laudon M, et al. (2017) Neu-P11, a novel MT1/MT2 agonist, reverses diabetes by suppressing the hypothalamic-pituitary-adrenal axis in rats. Eur. J. Pharmacol. 812: 225-233. doi: 10.1016/j.ejphar.2017.07.001.
124. Véniant MM, Hale C, Komorowski R, Chen MM, St Jean DJ, Fotsch C, et al. (2009) Time of the day for 11beta-HSD1 inhibition plays a role in improving glucose homeostasis in DIO mice. Diabetes Obes. Metab. 11 (2): 109-17. doi: 10.1111/j.1463-1326.2008.00911.x.
125. Dodd S, Skvarc DR, Dean OM, Anderson A, Kotowicz M, Berk M (2022) Effect of glucocorticoid and 11β-hydroxysteroid-dehydrogenase type 1 (11β-HSD1) in neurological and psychiatric disorders. Int. J. Neuropsychopharmacol. 25 (5): 387-398. doi: 10.1093/ijnp/pyac014.
126. Caughey S, Harris AP, Seckl JR, Holmes MC, Yau JL (2017) Forebrain-specific transgene rescue of 11β-HSD1 associates with impaired spatial memory and reduced hippocampal brain-derived neurotrophic factor mRNA levels in aged 11β-HSD1 deficient mice. J. Neuroendocrinol. 29 (1): 10.1111/jne.12447. doi: 10.1111/jne.12447.
127. Angelucci F, Veverova K, Katonová A, Vyhnalek M, Hort J (2023) Serum PAI-1/BDNF ratio is increased in alzheimer's disease and correlates with disease severity. ACS. Omega. 8 (39): 36025-36031. doi: 10.1021/acsomega.3c04076.
128. Numakawa T, Kajihara R (2023) Involvement of brain-derived neurotrophic factor signaling in the pathogenesis of stress-related brain diseases. Front. Mol. Neurosci. 16: 1247422. doi: 10.3389/fnmol.2023.1247422.
129. Pathak NM, Millar PJB, Pathak V, Flatt PR, Gault VA (2018) Beneficial metabolic effects of dietary epigallocatechin gallate alone and in combination with exendin-4 in high fat diabetic mice. Mol. Cell. Endocrinol. 460: 200-208. doi: 10.1016/j.mce.2017.07.024.
130. Al-Kuraishy HM, Jabir MS, Albuhadily AK, Al-Gareeb AI, Rafeeq MF (2023) The link between metabolic syndrome and Alzheimer disease: A mutual relationship and long rigorous investigation. Ageing Res. Rev. 91: 102084. doi: 10.1016/j.arr.2023.102084.
131. Ntamo Y, Jack B, Ziqubu K, Mazibuko-Mbeje SE, Nkambule BB, Nyambuya TM, et al. (2024) Epigallocatechin gallate as a nutraceutical to potentially target the metabolic syndrome: novel insights into therapeutic effects beyond its antioxidant and anti-inflammatory properties. Crit. Rev. Food. Sci. Nutr. 64 (1): 87-109. doi: 10.1080/10408398.2022.2104805.
132. Kupczyk D, Bilski R, Kozakiewicz M, Studzińska R, Kędziora-Kornatowska K, Kosmalski T, et al. (2022) 11β-HSD as a New target in pharmacotherapy of metabolic diseases. Int. J. Mol. Sci. 23 (16): 8984. doi: 10.3390/ijms23168984.
133. Schluessel S, Zhang W, Nowotny H, Bidlingmaier M, Hintze S, Kunz S, et al. (2023) 11-beta-hydroxysteroid dehydrogenase type 1 (HSD11B1) gene expression in muscle is linked to reduced skeletal muscle index in sarcopenic patients. Aging Clin. Exp. Res. 35 (12): 3073-3083. doi: 10.1007/s40520-023-02574-w.
134. Li H, Hu S, Wu R, Zhou H, Zhang K, Li K, et al. (2023) 11β-Hydroxysteroid dehydrogenase type 1 facilitates osteoporosis by turning on osteoclastogenesis through hippo signaling. Int. J. Biol. Sci. 19 (11): 3628-3639. doi: 10.7150/ijbs.82933.
135. Doig CL, Fletcher RS, Morgan SA, McCabe EL, Larner DP, Tomlinson JW, et al. (2017) 11β-HSD1 modulates the set point of brown adipose tissue response to glucocorticoids in male mice. Endocrinology 158 (6): 1964-1976.
136. Shi M, Watson E, Conlon M, Sanguansri L, Augustin MA (2022) Impact of co-delivery of EGCG and tuna oil within a broccoli matrix on human gut microbiota, phenolic metabolites and short chain fatty acids in vitro. Molecules 27 (3): 656. doi: 10.3390/molecules27030656.
137. Johnson JS, Opiyo MN, Thomson M, Gharbi K, Seckl JR, Heger A, et al. (2017) 11β-hydroxysteroid dehydrogenase-1 deficiency alters the gut microbiome response to Western diet. J. Endocrinol. 232 (2): 273-283. doi: 10.1530/JOE-16-0578.
138. Iwasaki Y, Takayasu S, Nishiyama M, Tsugita M, Taguchi T, Asai M, et al. (2008) Is the metabolic syndrome an intracellular Cushing state? Effects of multiple humoral factors on the transcriptional activity of the hepatic glucocorticoid-activating enzyme (11beta-hydroxysteroid dehydrogenase type 1) gene. Mol. Cell. Endocrinol. 285 (1-2): 10-8. doi: 10.1016/j.mce.2008.01.012.
139. Payne A, Taka E, Adinew GM, Soliman KFA (2023) Molecular mechanisms of the anti-inflammatory effects of epigallocatechin 3-gallate (EGCG) in LPS-Activated BV-2 microglia cells. Brain Sci. 13 (4): 632. doi: 10.3390/brainsci13040632.
140. Ma H, Yang L, Liu Y, Yan R, Wang R, Zhang P, et al. (2023) Butyrate suppresses atherosclerotic inflammation by regulating macrophages and polarization via GPR43/HDAC-miRNAs axis in ApoE-/- mice. PLoS One 18 (3): e0282685. doi: 10.1371/journal.pone.0282685.
141. Moniruzzaman M, Maiti AK, Chakraborty SB, Saha I, Saha NC (2022) Melatonin ameliorates lipopolysaccharide induced brain inflammation through modulation of oxidative status and diminution of cytokine rush in Danio rerio. Environ. Toxicol. Pharmacol. 96: 103983. doi: 10.1016/j.etap.2022.103983.
142. Burnatowska E, Wikarek A, Oboza P, Ogarek N, Glinianowicz M, Kocelak P, et al. (2023) Emotional eating and binge eating disorders and night eating syndrome in polycystic ovary syndrome-a vicious circle of disease: A systematic review. Nutrients 15 (2): 295. doi: 10.3390/nu15020295.
143. Anderson G (2024) Polycystic ovary syndrome pathophysiology: integrating systemic, CNS and circadian processes. Front. Biosc. (Landmark. Ed) 29 (1): 24. doi: 10.31083/j.fbl2901024.
144. Zhou Y, Cheng C, Baranenko D, Wang J, Li Y, Lu W (2018) Effects of acanthopanax senticosus on brain injury induced by simulated spatial radiation in mouse model based on pharmacokinetics and comparative proteomics. Int. J. Mol. Sci. 19 (1): 159. doi: 10.3390/ijms19010159.
145. Yao L, Lv J, Duan C, An X, Zhang C, Li D, et al. (2022) Armillaria mellea fermentation liquor ameliorates p-chlorophenylalanine-induced insomnia associated with the modulation of serotonergic system and gut microbiota in rats. J. Food. Biochem. 46 (2): e14075. doi: 10.1111/jfbc.14075.
146. Zheng Y, Deng Y, Gao JM, Lv C, Lang LH, Shi JS, et al. (2020) Icariside II inhibits lipopolysaccharide-induced inflammation and amyloid production in rat astrocytes by regulating IKK/IκB/NF-κB/BACE1 signaling pathway. Acta. Pharmacol. Sin. 41 (2): 154-162. doi: 10.1038/s41401-019-0300-2.
147. Cheng H, Zhang D, Wu J, Liu J, Zhou Y, Tan Y, et al. (2023) Interactions between gut microbiota and polyphenols: A mechanistic and metabolomic review. Phytomedicine 119: 154979. doi: 10.1016/j.phymed.2023.154979.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


