2-Hydroxymelatonin confers tolerance against combined cold and drought stress in tobacco, tomato, and cucumber as a potent anti-stress compound in the evolution of land plants
Functional role of 2-hydroxymelatonin in plants
Abstract
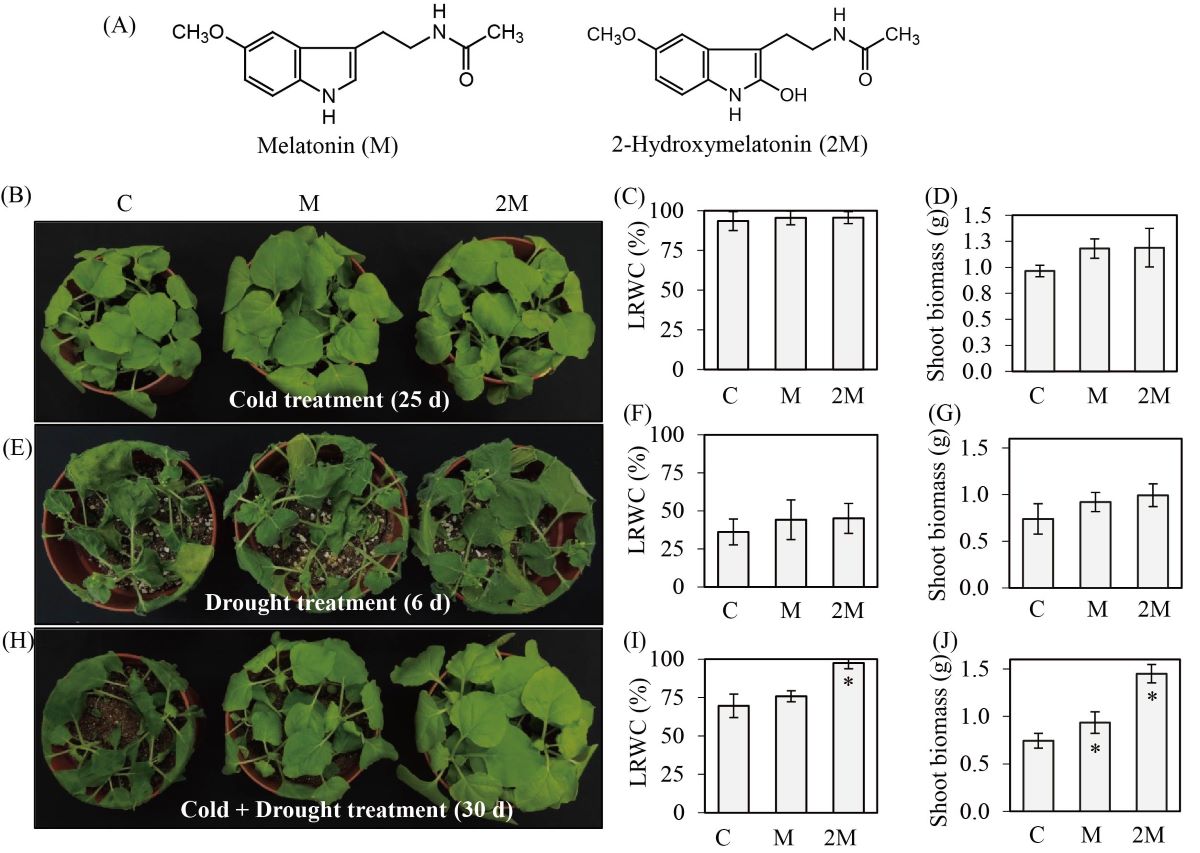
Melatonin (M) is an endogenous molecule found ubiquitously in animals and plants that helps maintain various biological functions. Unlike animals, plants preferentially synthesize 2-hydroxymelatonin (2M) over M, but the biological functions of 2M remain largely unknown. Here, we found that exogenous foliar application of 2M conferred tolerance against combined cold and drought stress in tobacco (Nicotiana benthamiana), tomato (Solanum lycopersicum L. cv. Micro-Tom), and cucumber (Cucumis sativus L. cv. Baecdadaki), whereas no such tolerance was observed against these stresses applied individually. Accordingly, endogenous 2M was induced in tobacco and tomato leaves in response to combined stress, whereas M levels remained unchanged in tobacco leaves and decreased in tomato leaves. After challenging tobacco and tomato leaves with prohexadione-calcium, an inhibitor of 2M synthesis, 2M levels decreased and led to hypersensitivity to combined stress. Because the gene encoding 2M is found only in land plants, and is absent in cyanobacteria and algae, we propose that 2M may have evolved as aquatic plants invaded land to overcome the stressors of virgin terrestrial environments, such as cold and drought.
References
2. Byeon Y, Lee K, Park YI, Park S, Back K (2013) Molecular cloning and functional analysis of serotonin N-acetyltransferase from the cyanobacterium Synechocystis sp. PCC 6803. J. Pineal Res. 55: 371-376.
3. Reiter RJ, Tan DX, Sharma R (2018) Historical perspective and evaluation of the mechanisms by which melatonin mediates seasonal reproduction in mammals. Melatonin Res. 1: 59-77.
4. Reiter RJ, Tan DX, Zhou Z, Cruz MHC, Fuentes-Broto L, Galano A (2015) Phytomelatonin: assisting plants to survive and thrive. Molecules. 20: 7396-7437.
5. Hardeland R (2016) Melatonin in plants – diversity of levels and multiplicity of functions. Front. Plant Sci. 7: 198.
6. Arnao MB, Hernández-Ruiz J (2019) Melatonin: a new plant hormone and/or a plant master regulator? Trends Plant Sci. 24: 38-48.
7. Wei W, Li QT, Chu YN, Reiter RJ, Yu XM, Zhu DH, Zhang WK, Ma B, Lin Q, Zhang JS, Chen SY (2015) Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp Bot. 66: 695-707.
8. Zhao H, Su T, Huo L, Wei H, Jiang Y, Xu L, Ma F (2015) Unveiling the mechanism of melatonin impacts on maize seedling growth: sugar metabolism as a case. J. Pineal Res. 59: 255-266.
9. Byeon, Y. & Back, K (XXX). Low melatonin production by suppression of either serotonin N-acetyltransferase or N-acetylserotonin methyltransferase causes seedling growth retardation with yield penalty, abiotic stress susceptibility, and enhanced coleoptile growth in rice under anoxic conditions. J. Pineal Res. 60: 411-421.
10. Lee HY, Byeon Y, Tan DX, Reiter RJ, Back K (2015) Arabidopsis serotonin N-acetyltransferase knockout plants exhibit decreased melatonin and salicylic acid levels resulting in susceptibility to an avirulent pathogen. J. Pineal Res. 58: 291-299.
11. Zhao H, Xu L, Su T, Jiang Y, Hu L, Ma F (2015) Melatonin regulates carbohydrate metabolism and defenses against Pseudomonas syringae pv. tomato DC3000 infection in Arabidopsis thaliana. J. Pineal Res. 59: 109-119.
12. Shi H, Tan DX, Reiter RJ, Ye T, Yang F, Chan Z (2015) Melatonin induces class A1 heat shock factors (HSFA1s) and their possible involvement of thermotolerance in Arabidopsis. J. Pineal Res. 58: 335-342.
13. Liang C, Zheng G, Li W, Wang Y, Hu B, Wang H, Wu H, Q Y, Zhu XG, Tan DX, Chen SY, Chu C (2015) Melatonin delays leaf senescence and enhances salt stress tolerance in rice. J. Pineal Res. 59: 91-101.
14. Dan Y, Zhang S, Zhong H, Yi H, Sainz MB (2015) Novel compounds that enhance Agrobacterium-mediated plant transformation by mitigating oxidative stress. Plant Cell Rep. 34: 291-309.
15. Lee HY, Back K (2018) Melatonin plays a pivotal role in conferring tolerance against endoplasmic reticulum stress via mitogen-activated protein kinases and bZIP60 in Arabidopsis thaliana. Melatonin Res. 1: 93-107.
16. Byeon Y, Back K (2015) Molecular cloning of melatonin 2-hydroxylase responsible for 2-hydroxymelatonin production in rice (Oryza sativa). J. Pineal Res. 58: 343-351.
17. Byeon Y, Tan DX, Reiter RJ, Back K (2016) Predominance of 2-hydroxymelatonin over melatonin in plants. J. Pineal Res. 59: 448-454.
18. Rochfort S, Parker AJ, Dunshea FR (2008) Plant bioactives for ruminant health and productivity. Phytochemistry. 69: 299-322.
19. Lee HY, Back K (2016) Mitogen-activated protein kinase pathways are required for melatonin-mediated defense responses in plants. J. Pineal Res. 60: 411-421.
20. Lee HJ, Back K (2016) 2-Hydroxymelatonin promotes the resistance of rice plant to multiple simultaneous abiotic stresses (combined cold and drought). J. Pineal Res. 61: 303-316.
21. Turner NC (1981) Techniques and experimental approaches for the measurement of plant water status. Plant Soil. 58: 339-366.
22. Wang X, Vignjevic M, Jiang D, Jacobsen S, Wollenweber B (2014) Improved tolerance to drought stress after anthesis due to priming before anthesis in wheat (Triticum aestivum L.) var. Vinjett. J. Exp. Bot. 65: 6441-6456.
23. Du Z, Bramlage WJ (1992) Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 40: 1566-1570.
24. Byeon Y, Lee HY, Back K (2015) Chloroplastic and cytoplasmic overexpression of sheep serotonin N-acetyltransferase in transgenic rice plants is associated with low melatonin production despite high enzyme activity. J. Pineal Res. 58: 461-469.
25. Otani M, Yoon JM, Park SH, Asami T, Nakajima M (2010) Screening and characterization of an inhibitory chemical specific to Arabidopsis gibberellin 2-oxidases. Bioorg. Med. Chem. Lett. 20: 4259-4262.
26. Lerner AB, Case JD, Takahashi Y (1958) Isolation of melatonin, a pineal factor that lightness melanocytes. J. Am. Soc. 80: 2587.
27. Kawai Y, Ono E and Mizutani M (2014) Evolution and diversity of the 2-oxoglutarate-dependent dioxygenase superfamily in plants. Plant J. 78: 328-343.
28. Kato M (2010) Evolution of primitive land plants: a review. Bull. Natl. Mus. Nat. Sci., Ser. B. 36: 1-11.
29. Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci. 11: 15-19.
30. Atkinson NJ, Urwin PE (2012) The interaction of plant biotic and abiotic stresses: from genes to the field. J. Exp. Bot. 63: 3523-3543.
31. Su L, Dai Z, Li S, Xin H (2015) A novel system for evaluating drought-cold tolerance of grapevines using chlorophyll fluorescence. BMC Plant Biol. 15: 82.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


