CREB1 spatio-temporal dynamics within the rat pineal gland
CREB1 dynamics in the rat pineal gland
Abstract
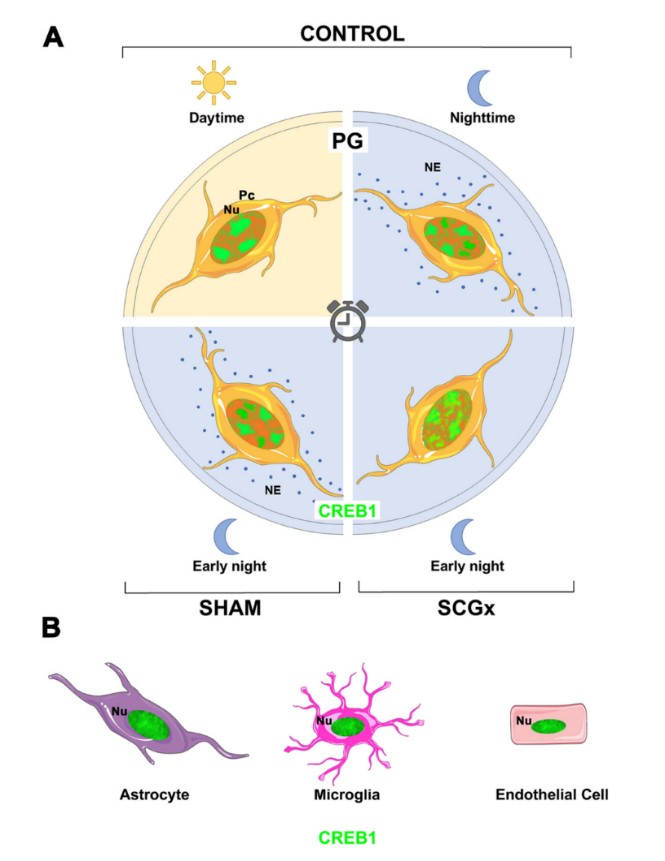
In the rat pineal gland (PG), cyclic AMP responsive element-binding protein 1 (CREB1) participates in the nocturnal melatonin synthesis that rhythmically modulates physiology and behavior. Phosphorylation of CREB1 is one of the key regulatory steps that drives pineal transcription. The spatio-temporal dynamics of CREB1 itself in the different PG cell types have not yet been documented. In this study we analyzed total CREB1 in the rat PG via Western blot and fluorescence immunohistochemistry followed by confocal laser-scanning microscopy and quantitative analysis. Total CREB1 levels remained constant in the PG throughout the light:dark cycle. The distribution pattern of nuclear CREB1 did vary among PG cell types. Pinealocytes emerged to have discrete CREB1 domains within their nucleoplasm that were especially distinct. The number, size, and location of CREB1 foci fluctuated among pinealocytes, within the same PG and among Zeitgeber times (ZTs). A significantly larger dispersion of CREB1-immunoreactive nuclear sites was found at night than during the day. However, the overall transcription activity was mostly conserved between the light and dark phases, as shown by the expression of a particular phosphorylated form of the RNA polymerase II (RNAPII-pSer5CTD). Suppression of the nocturnal norepinephrine pulse by chronic bilateral superior cervical ganglionectomy increased CREB1 dispersion in pinealocyte nuclei at early night, as compared to sham-derived cells. In addition, differences in CREB1 distribution were found between sham-operated and non-operated rats at ZT14. Together, these data suggest that in mature pinealocytes, nuclear CREB1 is subjected to a dynamic spatio-temporal distribution. Further studies are necessary to elucidate the underlying mechanisms and to understand the impact of CREB1 reorganization in the pineal transcriptome.
References
2. Maronde E, Stehle JH (2007) The mammalian pineal gland: known facts, unknown facets. Trends Endocrinol. Metab. 18: 142-149.
3. Lee BH, et al. (2020) Two indoleamines are secreted from rat pineal gland at night and act on melatonin receptors but are not night hormones. J. Pineal Res. 68: e12622.
4. Simonneaux V, Ribelayga C (2003) Generation of the melatonin endocrine message in mammals: a review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol. Rev. 55: 325-395.
5. Borjigin J, Zhang LS, Calinescu AA (2012) Circadian regulation of pineal gland rhythmicity. Mol. Cell. Endocrinol. 349: 13-19.
6. Stehle JH, et al. (1993) Adrenergic signals direct rhythmic expression of transcriptional repressor CREM in the pineal gland. Nature 365: 314-320.
7. Maronde E, et al. (1999) Transcription factors in neuroendocrine regulation: rhythmic changes in pCREB and ICER levels frame melatonin synthesis. J. Neurosci. 19: 3326-3336.
8. Maronde E, Schomerus C, Stehle JH, Korf HW (1997) Control of CREB phosphorylation and its role for induction of melatonin synthesis in rat pinealocytes. Biol. Cell 89: 505-511.
9. Rath MF, Rohde K, Klein DC, Møller M (2013) Homeobox genes in the rodent pineal gland: roles in development and phenotype maintenance. Neurochem. Res. 38: 1100-1112.
10. Rohde K, Hertz H, Rath MF (2019) Homeobox genes in melatonin-producing pinealocytes: Otx2 and Crx act to promote hormone synthesis in the mature rat pineal gland. J. Pineal Res. 66: e12567.
11. Hertz H, et al. (2020) The Lhx4 homeobox transcript in the rat pineal gland: Adrenergic regulation and impact on transcripts encoding melatonin-synthesizing enzymes. J. Pineal Res. 68: e12616.
12. Lonze BE, Ginty DD (2002) Function and regulation of CREB family transcription factors in the nervous system. Neuron 35: 605-623.
13. Montminy M, Bilezikjian L (1987) Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature 328: 175-178.
14. Mayr B, Montminy M (2001) Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2: 599-609.
15. Belgacem YH, Borodinsky LN (2017) CREB at the crossroads of activity-dependent regulation of nervous system development and function. Adv. Exp. Med. Biol. 1015: 19-39.
16. Tamotsu S, Schomerus C, Stehle JH, Roseboom PH, Korf HW (1995) Norepinephrine-induced phosphorylation of the transcription factor CREB in isolated rat pinealocytes: an immunocytochemical study. Cell Tissue Res. 282: 219-226.
17. Hagiwara M, et al. (1993) Coupling of hormonal stimulation and transcription via the cyclic AMP-responsive factor CREB is rate limited by nuclear entry of protein kinase A. Mol. Cell. Biol. 13: 4852-4859.
18. Sakamoto K, Karelina K, Obrietan K (2011) CREB: a multifaceted regulator of neuronal plasticity and protection. J. Neurochem. 116: 1-9.
19. Schumacher MA, Goodman RH, Brennan RG (2000) The structure of a CREB bZIP.somatostatin CRE complex reveals the basis for selective dimerization and divalent cation-enhanced DNA binding. J. Biol. Chem. 275: 35242-35247.
20. Craig JC, et al. (2001) Consensus and variant cAMP-regulated enhancers have distinct CREB-binding properties. J. Biol. Chem. 276: 11719-11728.
21. Maronde E, et al. (1999) CREB phosphorylation and melatonin biosynthesis in the rat pineal gland: involvement of cyclic AMP dependent protein kinase type II. J. Pineal Res. 27: 170-182.
22. Koch M, Mauhin V, Stehle JH, Schomerus C, Korf HW (2003) Dephosphorylation of pCREB by protein serine/threonine phosphatases is involved in inactivation of Aanat gene transcription in rat pineal gland. J. Neurochem. 85: 170-179 .
23. Roseboom PH, Klein DC (1995) Norepinephrine stimulation of pineal cyclic AMP response element-binding protein phosphorylation: primary role of a beta-adrenergic receptor/cyclic AMP mechanism. Mol. Pharmacol. 47: 439-449.
24. Baler R, Covington S, Klein DC (1997) The rat arylalkylamine N-acetyltransferase gene promoter. cAMP activation via a cAMP-responsive element-CCAAT complex. J. Biol. Chem. 272: 6979-6985.
25. Baler R, Covington S, Klein DC (1999) Rat arylalkylamine N-acetyltransferase gene: upstream and intronic components of a bipartite promoter. Biol. Cell 91: 699-705.
26. Klein DC (2007) Arylalkylamine N-acetyltransferase: "the Timezyme". J. Biol. Chem. 282: 4233-4237.
27. Bailey MJ, et al. (2009) Night/day changes in pineal expression of >600 genes: central role of adrenergic/cAMP signaling. J. Biol. Chem. 284: 7606-7622.
28. Coon SL, et al. (2019) Single cell sequencing of the pineal gland: the next chapter. Front. Endocrinol. 10: 590.
29. Hartley SW, et al. (2015) Neurotranscriptomics: the effects of neonatal stimulus deprivation on the rat pineal transcriptome. PLoS One 10: e0137548 .
30. Mays JC, et al. (2018) Single-cell RNA sequencing of the mammalian pineal gland identifies two pinealocyte subtypes and cell type-specific daily patterns of gene expression. PLoS One 13: e0205883.
31. Chang E, et al. (2020) Resource: a multi-species multi-timepoint transcriptome database and webpage for the pineal gland and retina. J. Pineal Res. 69: e12673.
32. Castro AE, et al. (2015) Expression and cellular localization of the transcription factor NeuroD1 in the developing and adult rat pineal gland. J. Pineal Res. 58: 439-451.
33. Rath MF, et al. (2016) Melatonin synthesis: acetylserotonin O-methyltransferase (ASMT) is strongly expressed in a subpopulation of pinealocytes in the male rat pineal gland. Endocrinology 157: 2028-2040.
34. Ibañez Rodriguez MP, et al. (2018) Differential response of pineal microglia to surgical versus pharmacological stimuli. J. Comp. Neurol. 526: 2462-2481.
35. Ibañez Rodriguez MP, Noctor SC, Muñoz EM (2016) Cellular basis of pineal gland development: emerging role of microglia as phenotype regulator. PLoS One 11: e0167063.
36. Møller M, Baeres FM (2002) The anatomy and innervation of the mammalian pineal gland. Cell Tissue Res. 309: 139-150.
37. Cremer T, et al. (2020) The interchromatin compartment participates in the structural and functional organization of the cell nucleus. Bioessays 42: e1900132.
38. Corpet A, et al. (2020) PML nuclear bodies and chromatin dynamics: catch me if you can! Nucleic Acids Res. 48: 11890-11912.
39. Liu Z, Tjian R (2018) Visualizing transcription factor dynamics in living cells. J. Cell Biol. 217: 1181-1191.
40. Stortz M, Presman DM, Pecci A, Levi V (2021) Phasing the intranuclear organization of steroid hormone receptors. Biochem. J. 478: 443-461.
41. Arnett-Mansfield RL, et al. (2007) Focal subnuclear distribution of progesterone receptor is ligand dependent and associated with transcriptional activity. Mol. Endocrinol. 21: 14-29.
42. Fejes-Toth G, Pearce D, Naray-Fejes-Toth A (1998) Subcellular localization of mineralocorticoid receptors in living cells: effects of receptor agonists and antagonists. Proc. Natl. Acad. Sci. USA 95: 2973-2978.
43. Grande MA, van der Kraan I, de Jong L, van Driel R (1997) Nuclear distribution of transcription factors in relation to sites of transcription and RNA polymerase II. J. Cell Sci. 110: 1781-1791.
44. Martins VR, et al. (1991) Demonstration by confocal microscopy that unliganded overexpressed glucocorticoid receptors are distributed in a nonrandom manner throughout all planes of the nucleus. Mol. Endocrinol. 5: 217-225.
45. Wagh K, Garcia DA, Upadhyaya A (2021) Phase separation in transcription factor dynamics and chromatin organization. Curr. Opin. Struct. Biol. 71: 148-155.
46. Loh D, Reiter RJ (2021) Melatonin: regulation of biomolecular condensates in neurodegenerative disorders. Antioxidants (Basel) 10: 1483.
47. Loh D, Reiter RJ (2023) Light, water, and melatonin: the synergistic regulation of phase separation in dementia. Int. J. Mol. Sci. 24: 5835.
48. Sugo N, et al. (2015) Single-molecule imaging reveals dynamics of CREB transcription factor bound to its target sequence. Sci. Rep. 5: 10662.
49. Kitagawa H, et al. (2017) Activity-dependent dynamics of the transcription factor of cAMP-response element binding protein in cortical neurons revealed by single-molecule imaging. J. Neurosci. 37: 1-10.
50. Mayr BM, Guzman E, Montminy M (2005) Glutamine rich and basic region/leucine zipper (bZIP) domains stabilize cAMP-response element-binding protein (CREB) binding to chromatin. J. Biol. Chem. 280: 15103-15110.
51. Savastano LE, et al. (2010) A standardized surgical technique for rat superior cervical ganglionectomy. J. Neurosci. Methods 192: 22-33.
52. Madhani SI, Klein DC, Muñoz EM, Savastano LE (2022) Surgical techniques and nuances for superior cervical ganglionectomy and decentralization in rats. Methods Mol. Biol. 2550: 53-62.
53. Welinder C, Ekblad L (2011) Coomassie staining as loading control in Western blot analysis. J. Proteome Res. 10: 1416-1419.
54. Bass JJ, et al. (2017) An overview of technical considerations for Western blotting applications to physiological research. Scand. J. Med. Sci. Sports 27: 4-25.
55. Taylor SC, Berkelman T, Yadav G, Hammond M (2013) A defined methodology for reliable quantification of Western blot data. Mol. Biotechnol. 55: 217-226.
56. Otsu N. (1979) A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 9: 62-66.
57. Yang Q, Yu W, Han X (2019) Overexpression of microRNA101 causes antitumor effects by targeting CREB1 in colon cancer. Mol. Med. Rep. 19: 3159-3167.
58. Nasca C, et al. (2017) Role of the astroglial glutamate exchanger xCT in ventral hippocampus in resilience to stress. Neuron 96: 402-413.e5.
59. Kitagawa Y, et al. (2017) Guidance of regulatory T cell development by Satb1-dependent super-enhancer establishment. Nat. Immunol. 18: 173-183.
60. Farias Altamirano LE, et al. (2022) Spatio-temporal dynamics of nuclear CREB1: what does it mean? bioRxiv 10.1101/2022.06.26.497665.
61. Cha-Molstad H, Keller DM, Yochum GS, Impey S, Goodman RH (2004) Cell-type-specific binding of the transcription factor CREB to the cAMP-response element. Proc. Natl. Acad. Sci. USA 101: 13572-13577.
62. Impey S, et al. (2004) Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell 119: 1041-1054.
63. Schomerus C, Maronde E, Laedtke E, Korf HW (1996) Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP) induce phosphorylation of the transcription factor CREB in subpopulations of rat pinealocytes: immunocytochemical and immunochemical evidence. Cell Tissue Res. 286: 305-313.
64. Schomerus C, Laedtke E, Korf HW (2003) Norepinephrine-dependent phosphorylation of the transcription factor cyclic adenosine monophosphate responsive element-binding protein in bovine pinealocytes. J. Pineal Res. 34: 103-109.
65. Birk UJ (2019) Super-resolution microscopy of chromatin. Genes (Basel) 10: 493.
66. Nozaki T, et al. (2017) Dynamic organization of chromatin domains revealed by super-resolution live-cell imaging. Mol. Cell 67: 282-293-e287.
67. Muñoz EM (2018) Microglia-precursor cell interactions in health and in pathology. Biocell 42: 41-45.
68. Muñoz EM (2022) Microglia in circumventricular organs: the pineal gland example. ASN Neuro 14: 17590914221135697.
69. Phatnani HP, Greenleaf AL (2006) Phosphorylation and functions of the RNA polymerase II CTD. Genes Dev. 20: 2922-2936.
70. Jasnovidova O, Stefl R (2013) The CTD code of RNA polymerase II: a structural view. Wiley Interdiscip. Rev. RNA 4: 1-16.
71. Klein DC (2007) The pineal gene expression party: who's the surprise guest? Endocrinology 148: 1463-1464.
72. Chik CL, et al. (2007) Histone H3 phosphorylation in the rat pineal gland: adrenergic regulation and diurnal variation. Endocrinology 148: 1465-1472.
73. Ho AK, et al. (2007) Acetylation of histone H3 and adrenergic-regulated gene transcription in rat pinealocytes. Endocrinology 148: 4592-4600.
74. Ho AK, Chik CL (2010) Modulation of Aanat gene transcription in the rat pineal gland. J. Neurochem. 112: 321-331.
75. Koike N, et al. (2012) Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science 338: 349-354.
76. Takahashi JS (2017) Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 18: 164-179.
77. da Silveira Cruz-Machado S, Pinato L, Tamura EK, Carvalho-Sousa CE, Markus RP (2012) Glia-pinealocyte network: the paracrine modulation of melatonin synthesis by tumor necrosis factor (TNF). PLoS One 7: e40142.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


