Effect of iron on rat serum melatonin levels under different light/dark cycle patterns
Iron and serum melatonin
Abstract
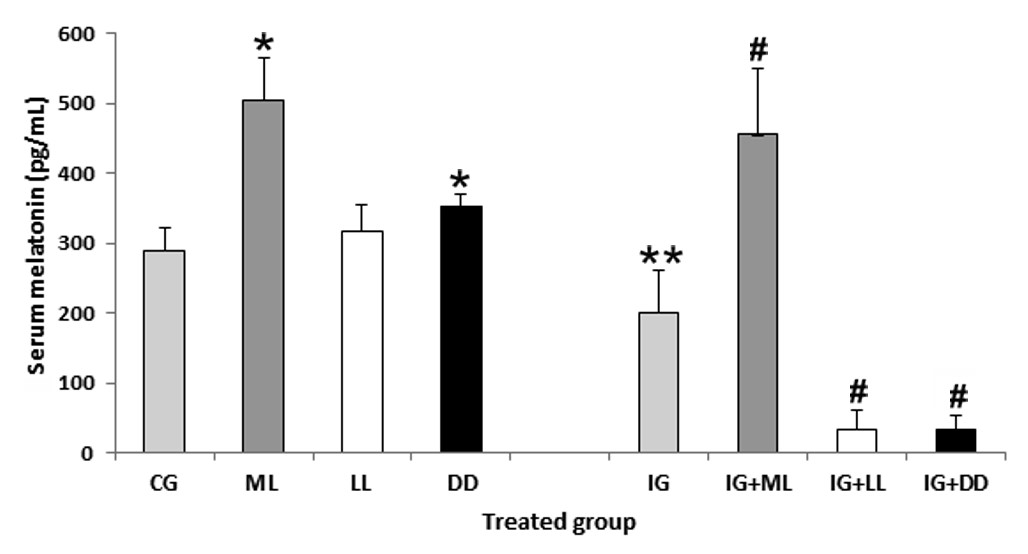
Exposure to constant light or darkness for long periods has diverse effects on circadian physiology. Iron (Fe) overloading promotes oxidative stress and causes alterations in cellular structure and function in animals and humans. The aim of this study is to evaluate the interactions among serum melatonin (ML), photoperiod manipulation, and Fe overloading in rats. The results showed that constant darkness exposure for 15 days significantly increased serum ML levels (up to 22%) while the constant light exposure failed to reduce the serum ML level compared to the normal light/dark cycle treated rats. The lost serum ML level usually from the pineal gland under the long term of constant light exposure may be compensated by ML generated by other organs which adapted to the situation. Also, Fe overloading decreased ML production due to this molecule being consumed to scavenge the free radicals induced by the Fe overloading. In addition, we observed interactions among constant light or darkness exposure, Fe overloading and serum ML level. Overall, our results support the hypothesis of ML as scavenging molecule; it may be an effective therapeutic tool in iron-induced oxidative stress.
References
2. Poeggeler B (1993) Melatonin and the light-dark zeitgeber in vertebrates, invertebrates and unicellular organisms. Experientia 49: 611-613.
3. Pandi-Perumal SR, Srinivasan V, Maestroni GJM, Cardinali DP, Poeggeler B, Hardeland R (2006) Melatonin-Nature’s most versatile biological signal? FEBS J. 273: 2813-2838.
4. Tan DX, Hardeland R, Manchester LC, Paredes SD, Korkmaz A, Sainz RM, Mayo JC, Fuentes-Broto L, Reiter RJ (2010) The changing biological roles of melatonin during evolution: from an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev. 85: 607-623.
5. Weil ZM, Borniger JC, Cisse YM, Abi Salloum BA, Nelson RJ (2015) Neuroendocrine control of photoperiodic changes in immune function. Front. Neuroendocrinol. 37: 108-118.
6. Naguib N, Gottumukkala V, Goldstein PA (2007) Melatonin and anesthesia: a clinical perspective. J. Pineal Res. 42: 12-21.
7. Wiechmann AF, Sherry DM (2013) Role of melatonin and its receptors in the vertebrate retina. Int. Rev. Cell Mol. Biol. 300: 211-242.
8. Zawilska JB (1996) Melatonin as a chemical indicator of environmental light-dark cycle. Acta Neurobiol. Exp. 56: 757-767.
9. Arendt J, Skene DJ (2005) Melatonin as a chronobiotic. Sleep Med. Rev. 9: 25-39.
10. Bello-Caraballo N, Cogo Pagella J, Iodice O, Cervino CO (2018) Efectos de la combinación de melatonina con clorhidrato de ketamina sobre regímenes estándar de anestesia en ratas. Rev. Argent. Anestesiol. 76: 75-84.
11. Huether G (1993) The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia 49 (8): 665-70. doi: 10.1007/BF01923948.
12. Kvetnoy I (1999) Extrapineal melatonin: location and role within diffuse neuroendocrine system. Histochem. J. 31: 1–12.
13. Acuña-Castroviejo D, Escames G, Venegas C, Diaz‑Casado ME, Lima‑Cabello E, Lopez LC, Rosales-Corral S, Tan DX, Reiter RJ (2014) Extrapineal melatonin: sources, regulation, and potential functions. Cell. Mol. Life Sci. 71: 2997-3025.
14. Raikhlin NT, Kvetnoy IM, Tolkachev VN (1975) Melatonin may be synthesized in enterochromaffin cells. Nature 255: 344–345.
15. Bubenik GA, Pang SF, Hacker RR, Smith PS (1996) Melatonin concentrations in serum and tissues of porcine gastrointestinal tract and their relationship to the intake and passage of food. J. Pineal Res. 21: 251–256.
16. Vician M, Zeman M, Herichova I, Jurani M, Blazicek P, Matis P (1999) Melatonin content in plasma and large intestine of patients with colorectal carcinoma before and after surgery. J. Pineal Res. 27: 164–169.
17. Sanchez-Hidalgo M, de la Lastra CA, Carrascosa-Salmoral MP, Naranjo MC, Gomez-Corvera A, Caballero B, Guerrero JM (2009) Age-related changes in melatonin synthesis in rat extrapineal tissues. Exp. Gerontol. 44: 328–334.
18. Garcia JJ, Lopez-Pingarron L, Almeida-Souza P, Tres A, Escudero P, Garcia-Gil FA, Tan DX, Reiter RJ, Ramirez JM, Bernal-Perez M (2014) Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: a review. J. Pineal Res. 56: 225-237.
19. Reiter RJ, Tan DX, Mayo JC, Sainz RM, Leon J, Czarnocki Z (2003) Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 50: 1129-1146.
20. Acuña-Castroviejo D, Escames G, Rodriguez MI, Lopez LC (2007) Melatonin role in the mitocondrial function. Front. Biosci. 12: 947-963.
21. Skulachev VP (2009) New data on biochemical mechanism of programmed senescence of organisms and antioxidant defense of mitochondria. Biochemistry-Moscow 74: 1400-1403.
22. Süzen S, Atayik MC, Sirinzade H, Entezari B, Gurer-Orhan H, Cakatay U (2022) Melatonin and redox homeostasis. Melatonin Res. 5 (3): 304-324. doi:https://doi.org/https://doi.org/10.32794/mr112500134.
23. Häubner N, Sylvander P, Vuori K, Snoeijs P (2014) Abiotic stress modifies the synthesis of alpha-tocopherol and beta-carotene in phytoplankton species. J. Phycol. 50: 753–759. https://doi.org/10.1111/jpy.12198
24. Cervino CO, Hernando M (2015) La melatonina como agente antioxidante: análisis de estrés oxidativo en un modelo animal con exposición al hiero. Rev. de la Facultad de Ciencias Exactas, Químicas y Naturales (UM) 13: 89-103.
25. Piloni NE, Reiteri M, Hernando MP, Cervino CO, Puntarulo S (2017) Differential effect of acute iron overload on oxidative status and antioxidant content in regions of rat brain. Toxicol. Pathol. 45: 1067-1076.
26. Hernando M, Iodice O, Cervino CO (2019) Preacondicionamiento con hierro: efecto sobre la respuesta oxidativa en cerebro de ratas. Rev. Investigaciones Científicas de la Univ. de Morón 4: 37-49.
27. Hernando M, Piloni NE, Cervino CO, Puntarulo S (2019) Chapter 4.The role of melatonin on intracellular oxidative stress. Interaction with Fe-overload. Advances in Medicine and Biology, ed. Berhardt LV (Nova Science Publishers Inc, New York), pp. 63-84.
28. Galleano M, Puntarulo S (1994) Effect of mild iron overload on liver and kidney lipid peroxidation. Braz. J. Med. Biol. Res. 27: 2349–2358.
29. Puntarulo S (2005) Iron, oxidative stress and human health. Mol. Asp. Med. 26: 299-312.
30. Fahn S, Cohen G (1992) The oxidant stress hypothesis in Parkinson's disease: evidence supporting it. Ann. Neurol. 32: 804-812.
31. Halliwell B, Gutteridge JM (1992) Biologically relevant metal ion-dependent hydroxyl radical generation. An update. FEBS Lett. 307: 108-112.
32. Halliwell B, Gutteridge JMC (1984) Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 219: 1–14.
33. Fraga CG, Oteiza PI (2002) Iron toxicity and antioxidant nutrients. Toxicology 180: 23-32.
34. Hirschhorn T, Stockwell BR (2019) The Development of the concept of ferroptosis. Free Radic. Biol. Med. 133: 130–143. doi:10.1016/j.freeradbiomed.2018.09.043.
35. Ren J-X, Sun X, Yan X-L, Guo Z-N and Yang Y (2020) Ferroptosis in neurological diseases. Front. Cell. Neurosci. 14: 218. doi: 10.3389/fncel.2020.00218
36. Limson J, Nyokong T, Daya S (1998) The interaction of melatonin and its precursors with aluminium, cadmium, copper, iron, lead, and zinc: an adsorptive voltammetric study. J. Pineal Res. 24: 15–21.
37. Gulcin I, Buyukokuroglu ME, Kufrevioglu OI (2003) Metal chelating and hydrogen peroxide scavenging effects of melatonin. J. Pineal Res. 34: 278-281.
38. González PL, Piloni NE, Puntarulo S (2012) Iron overload and lipid peroxidation in biological systems. Lipid Peroxidation, ed. Catala A. (IntechOpen) pp. 89-109. https://doi.org/10.5772/2929.
39. Galleano M, Puntarulo S (1995) Role of antioxidants on the erythrocytes resistance to lipid peroxidation after acute iron overload in rats. Biochim. Biophys. Acta 1271: 321-326.
40. Pablos MI, Agapito MT, Recio JM, Pérez-Gallardo L, Córdova MD, Mori JO (1993) Effect of iron and estrogen on melatonin secretion by the chicken pineal gland. Neurosci. Lett. 159: 211-214.
41. Hayter CL, Bishop GM, Robinson SR (2004) Pharmacological but not physiological concentrations of melatonin reduce iron-induced neuronal death in rat cerebral cortex. Neurosci. Lett. 362: 182-184.
42. Romero A, Ramos E, de Los Ríos C, Egea J, Del Pino J, Reiter RJ (2014) A review of metal-catalyzed molecular damage: protection by melatonin. J. Pineal Res. 56: 343-370.
43. Mantovani M, Kaster MP, Pertile R, Calixto JB, Rodrigues AL, Santos AR (2006) Mechanisms involved in the antinociception caused by melatonin in mice. J. Pineal Res. 41 (4): 382-389. doi: 10.1111/j.1600-079X.2006.00380.x.
44. Ayer RE, Sugawara T, Chen W, Tong W, Zhang JH (2008) Melatonin decreases mortality following severe subarachnoid hemorrhage. J. Pineal Res. 44 (2): 197-204. doi: 10.1111/j.1600-079X.2007.00508.x.
45. Pechlivanova D, Dzambazova E, Kolev G, Petkova Z, Tchekalarova J (2016) Effects of melatonin on stress-induced and diurnal variations of nociception in wistar and spontaneously hypertensive rats. Comptesrendus de l’Académiebulgare des Sciences 69 (9): 1223-1230.
46. Fukuhara C, Aguzzi J, Bullock N, Tosini G (2005) Effect of long-term exposure to constant dim light on the circadian system of rats. Neurosignals 14: 117-125.
47. Honma S, Kanematsu N, Katsuno Y, Homna K (1996) Persistence of circadian oscillation while locomotor activity and plasma melatonin levels became aperiodic under prolonged continuous light in the rat. Neurosci. Lett. 216: 49-52.
48. Herichová I, Zeman M, Stebelová K, Ravingerová T (2005) Effect of streptozotocin-induced diabetes on daily expression of per2 and dbp in the heart and liver and melatonin rhythm in the pineal gland of Wistar rat. Mol. Cell. Biochem. 270: 223–229.
49. Farhadi N, Gharghani M, Farhadi Z (2016) Effects of long-term light, darkness and oral administration of melatonin on serum levels of melatonin. Biomed. J. 39: 81-84.
50. Reiter RJ (1993) The melatonin rhythm-both clock and calendar. Experientia 49: 654-664.
51. Bruning A, Hölker F, Wolter C (2011) Artificial light at night: implications for early life stages development in four temperate freshwater fish species. Aquat. Sci 73: 143-152.
52. Vinogradova IA, Anisimov VN, Bukalev AV, Ilyukha VA, Khizhkin EA, Lotosh TA, Semenchenko AV, Zabezhinski MA (2009) Circadian disruption induced by light-at-night accelerates aging and promotes tumorigenesis in young but not in old rats. Aging 2: 82-92.
53. Ma WP, Cao J, Tian M, Cui M.H, Han HL, Yang YX, Xu L (2007) Exposure to chronic constant light impairs spatial memory and influences long-term depression in rats. Neurosci. Res. 59: 224-230.
54. Anisimov VN (2006) Light pollution, reproductive function and cancer risk. Neuroendocrinol. Lett. 27: 35-52.
55. Moore CB, Siopes TD (2000) Effects of lighting conditions and melatonin supplementation on the cellular and humoral immune responses in Japanese quail Coturnixcoturnix japonica. Gen. Comp. Endocrinol. 119: 95-104.
56. Oishi K, Shibusawa K, Kakazu H, Kuriyama T, Ohkura N, Machida K (2006) Extended light exposure suppresses nocturnal increases in cytotoxic activity of splenic natural killer cells in rats. Biol. Rhythm Res. 37: 29-35.
57. Bonmati-Carrion MA, Arguelles-Prieto R, Martinez-Madrid MJ, Reiter RJ, Hardeland R, Rol MA, Madrid JA (2014) Protecting the melatonin rhythm through circadian healthy light exposure. Int. J. Mol. Sci.15: 23448-23500.
58. Cho YM, Ryu SH, Lee BR, Kim KH, Lee E, Choi J (2015) Effects of artificial light at night on human health: A literature review of observational and experimental studies applied to exposure assessment. Chronobiol. Int. 32: 1-17.
59. Longcore T, Rich C (2004) Ecological light pollution. Front. Ecol. Environ. 2: 191-198.
60. Gaston KJ, Bennie J, Davies TW, Hopkins J (2013) The ecological impacts of nighttime light pollution: a mechanistic appraisal. Biol. Rev. 88: 912-927.
61. Tomás-Zapico C, Coto-Montes A, Martínez-Fraga J, Rodríguez-Colunga MJ, Tolivia D (2003) Effects of continuous light exposure on antioxidant enzymes, porphyric enzymes and cellular damage in the Harderian gland of the Syrian hamster. J. Pineal Res. 34: 60-68.
62. Baydaş G, Erçel E, Canatan H, Dönder E, Akyol A (2001), Effect of melatonin on oxidative status of rat brain, liver and kidney tissues under constant light exposure. Cell. Biochem. Funct.19: 37-41.
63. Escribano BM, Moreno A, Tasset I, Túnez I (2014) Impact of light/dark cycle patterns on oxidative stress in an adriamycin-induced nephropathy model in rats. PLoS ONE 9 (5): e97713. doi:10.1371/journal.pone.0097713.
64. Mochizuki M, Kuwabara T, Gery I (1988) Effects of continuous light exposure on the rat retina and pineal gland. Graefes Arch. Clin. Exp. Ophthalmol. 226: 346-352.
65. Logvinov, SV, Gerasimov AV, Kostiuchenko VP (2004) Ultrastructure of the pinealocytes in rats exposed to light and radiation. Morfologia 125: 71-75.
66. González MMC, Aston-Jones G (2006) Circadian regulation of arousal: Role of the noradrenergic locus coeruleus system and light exposure. Sleep 29: 1327-1336.
67. González MMC, Aston-Jones G (2008) Light deprivation damages monoamine neurons and produces a depressive behavioral phenotype in rats. Proc. Natl. Acad. Sci. USA 105: 4898-4903.
68. González MMC (2018) Dim light at night and constant darkness: Two frequently used lighting conditions that jeopardize the health and well-being of laboratory rodents. Front. Neurol. 9: 609. https://doi.org/10.3389/fneur.2018.00609.
69. Arendt J (2012) Biological rhythms during residence in polar regions. Chronobiol. Int. 29: 379-394.
70. López-González MA, Guerrero JM, Delgado F (1997) Presence of the pineal hormone melatonin in rat cochlea: its variations with lighting conditions. Neurosci. Lett. 238: 81-83.
71. Ayhan IC, Toyran N, Gundogan UN (2010) Exposure to continuous darkness leads to atypical symptoms of seasonal affective disorder in rats. Turk J. Med. Sci. 40: 271-277.
72. Cardinali DP, Pévet P (1998) Basic aspects of melatonin action. Sleep Med. Rev. 2: 175-190.
73. Hidalgo C, Carrasco MA, Muñoz P, Nuñez MT (2007) A Role for reactive oxygen/nitrogen species and iron on neuronal synaptic plasticity. Antiox. Redox Signal 9: 245-255.
74. Piloni N, Puntarulo S (2010) Iron role in the oxidative metabolism of animal and plant cells. Effect of iron overload. Metals in Biology Systems, Research Signpost, ed. Gimenez MS (Transworld Research Network, Trivandrum, Kerala, India), pp. 29-50.
75. Piloni N, Fernández V, Videla L, Puntarulo S (2013) Acute iron overload and oxidative stress in brain. Toxicology 314: 174-182.
76. Tan DX, Chen LD, Poeggeler B, Manchester LC, Reiter RJ (1993) Melatonin: apotent, endogenous hydroxyl radical scavenger. Endocr J. 1: 57–60.
77. Pieri C, Marra M, Moroni F, Recchioni R, Marcheselli F (1994) Melatonin: a peroxyl radical scavenger more effective than vitamin E. Life Sci. 55 (15): PL271–PL276. doi: 10.1016/0024-3205(94)00666-0. PMID: 7934611.
78. Reiter RJ (1996) Functional aspects of the pineal hormone melatonin in combating cell and tissue damage induced by free radicals. Eur. J. Endocrinol. 134: 412–420.
79. Tan DX, Manchester LC, Reiter RJ, Qi WB, Karbownik M, Calvo JR (2000) Significance of melatonin in antioxidative defense system: reactions and products. Biol Signals Recept. 9 (3-4): 137-159. doi: 10.1159/000014635.
80. Reiter RJ, Acuña-Castroviejo D, Tan DX, Burkhardt S (2001) Free radical-mediated molecular damage. Ann. N. Y. Acad. Sci. 939: 200–215.
81. Kotler M, Rodríguez C, Sáinz RM, Antolín I, Menéndez-Peláez A (1998) Melatonin increases gene expression for antioxidant enzymes in rat brain cortex. J. Pineal Res. 24: 83–89.
82. Lin AM, Ho LT (2000) Melatonin suppresses iron-induced neurodegeneration in rat brain. Free Radic. Biol. Med. 28: 904–911.
83. Othman AI, El-Missiry MA, Amer MA, Arafa M (2008) Melatonin controls oxidative stress and modulates iron, ferritin, and transferrin levels in adriamycin treated rats. Life Sci. 83 (15-16): 563-568 doi: 10.1016/j.lfs.2008.08.004.
84. Yasutake A, Hirayama K (2004) Effects of iron overload on hepatic and renal metallothionein levels in rats. J. Health Sci. 50: 372-378.
85. Abd Elkader MAE, Aly HH (2014) Protective effect of melatonin against iron overload-induced toxicity in rats. Int. J. Pharm. Pharm. Sci. 7: 116-121.
86. Fleet JC, Andrews GK, McCormick CC (1990) Iron-induced metallothionein in chick liver: rapid, rout-dependant effect independent of zinc status. J. Nutr.120: 1214-1222.
87. Günther T, Grossrau R, Höllriegl V, Vormann J (1991) Effects of Fe salicylate and Zn on metallothionein and lipid peroxidation in vivo. J. Trace Elem. Electrolytes Health. Dis. 5: 95-100.
88. Baran D, Paduraru I, Saramet A, Petrescu E, Haulica I (2000) Influence of light-dark cycle alteration on free radical level in rat CNS. Rom. J. Physiol. 37 (1-4): 23-38.
89. Subash S, Subramanian P, Sivaperumal R, Manivasagam T, Mohamed Essa M (2006) Constant light influences the circadian oscillations of circulatory lipid peroxidation, antioxidants and some biochemical variables in rats. Biol. Rhythm Res. 37: 471-477. doi: 10.1080/09291010600738692.
90. Túnez I, del Carmen Muñoz M, Feijoo M, Valdelvira ME, Rafael Muñoz-Castañeda J, Montilla P (2003) Melatonin effect on renal oxidative stress under constant light exposure. Cell. Biochem. Funct. 21 (1): 35-40.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


