Melatonin modulates the in vitro angiogenesis of granulosa cells collected from women with marital infertility for IVF
Melatonin effects on granulosa cells
Abstract
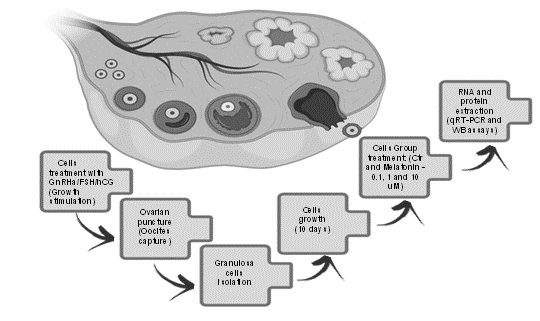
Melatonin concentration is several folds higher in the follicular fluid than that in blood suggesting an important role of this molecule on follicular physiology. However, the actions of melatonin on angiogenesis in granulosa cells are currently unknown. In this study, we have specifically investigated the potential effects of melatonin on the angiogenesis in granulosa cells from female individuals with marital infertility. Sixty patients who were submitted to the in vitrofertilization were included. The granulosa-luteal cells of these females were collected for cell culture. The cells were divided into four groups: a) vehicle (control); b) 0.1 µM melatonin; c) 1 µM melatonin; d) 10 µM melatonin treated groups, respectively. After a period of 10 days of culture, expression of genes involved in the angiogenesis signaling pathway were analyzed by Real-Time PCR and Western Blot assays. The results showed that the expressions of FGF1(fibroblast growth factor 1), IL1B (interleukin 1-beta), VEGFR-2(type 2 vascular-endothelial growth factor receptor), and TGFB1 (tumor growth factor 1- beta) were significantly upregulated in melatonin treated groups compared to the control. In contrast, the expressions of HIF-1A(hypoxia-inducing factor 1-alpha), FGF2 (fibroblastic growth factor 2), IGF-1(insulin-like growth factor 1), and VEGFA (vascular endothelial growth factor alpha) were significantly downregulated by melatonin compared to the control. The results suggest that melatonin modulates angiogenesis of granulosa cells from women with marital infertility. The underlining mechanism may relate to melatonin maintaining the homeostasis of VEGF, especially at a low dose of melatonin.
References
2. Dubocovich ML (2007) Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep Med. 8: 34-42.
3. Soares JM Jr, Masana MI, Erşahin C, Dubocovich ML (2003) Functional melatonin receptors in rat ovaries at various stages of the estrous cycle. J. Pharmacol. Exp. Ther. 306: 694-702.
4. Tamura H, Jozaki M, Tanabe M, Shirafuta Y, Mihara Y, Shinagawa M, Tamura I, Maekawa R, Sato S, Taketani T, Takasaki A, Reiter RJ (2020) Importance of melatonin in assisted reproductive technology and ovarian aging. Int. J. Mol. Sci. 21: 1135.
5. Brzezinski A, Seibel MM, Lynch HJ, Deng MH, Wurtman RJ (1987) Melatonin in human preovulatory follicular fluid. J. Clin. Endocrinol. Metab. 64: 865-7.
6. Minguini IP, Luquetti CM, Baracat MCP, Maganhin CC, Nunes CO, Simões RS, Veiga ECA, Cipolla Neto J, Baracat EC, Soares Junior JM (2019) Melatonin effects on ovarian follicular cells: a systematic review. Rev. Assoc. Med. Bras. 65: 1122-1127.
7. Coelho LA, Peres R, Amaral FG, Reiter RJ, Cipolla-Neto J (2015) Daily differential expression of melatonin-related genes and clock genes in rat cumulus-oocyte complex: changes after pinealectomy. J. Pineal. Res. 58: 490-499.
8. Pang Y, Zhao S, Sun Y, Jiang X, Hao H, Du W,Zhu H (2018). Protective effects of melatonin on the in vitro developmental competence of bovine oocytes. Anim. Sci. J. 89: 648–660.
9. Cheng L, Qin Y, Hu X, Ren L, Zhang C, Wang X, Wang W, Zhang Z, Hao J, Guo M, Wu Z, Tian J, An L (2019). Melatonin protects in vitro matured porcine oocytes from toxicity of Aflatoxin B1. J. Pineal Res. 66: e12543.
10. Nagahama Y (1997). 17, 20-dihydroxy-4-pregnen-3-one, a maturation-inducing hormone in fish oocytes: mechanisms of synthesis and action. Steroids 62: 190–196.
11. Sánchez-Ajofrín I, Martín-Maestro A, Medina-Chávez DA, Laborda-Gomariz JÁ, Peris-Frau P, Garde JJ, Soler AJ (2022). Melatonin rescues the development and quality of oocytes and cumulus cells after prolonged ovary preservation: An ovine in vitro model. Theriogenology 186: 1-11.
12. Kim MK, Park EA, Kim HJ, Choi WY, Cho JH, Lee WS, Cha KY, Kim YS, Lee DR, Yoon TK (2013). Does supplementation of in-vitro culture medium with melatonin improve IVF outcome in PCOS? Reprod. Biomed. Online 26: 22-29.
13. Tamanini C, De Ambrogi M (2004) Angiogenesis in developing follicle and corpus luteum. Reprod. Domest. Anim. 39: 206-216.
14. Alvarez-García V, González A,Alonso-González C, Martínez-Campa C, Cos S (2013) Regulation of vascular endothelial growth factor by melatonin in human breast cancer cells. J. Pineal. Res. 54: 373-380.
15. Ma Q, Reiter RJ, Chen Y (2020) Role of melatonin in controlling angiogenesis under physiological and pathological conditions. Angiogenesis 23: 91-104.
16. Reiter RJ, Tamura H, Tan DX, Xu XY (2014) Melatonin and the circadian system: contributions to successful female reproduction. Fertil. Steril. 102: 321-328.
17. Cipolla-Neto J, Amaral FGD (2018) Melatonin as a hormone: new physiological and clinical insights. Endocr. Rev. 39: 990-1028.
18. Cipolla-Neto J, Amaral FG, Soares JM Jr, Gallo CC, Furtado A, Cavaco JE, Gonçalves I, Santos CRA, Quintela T (2022) The crosstalk between melatonin and sex steroid hormones. Neuroendocrinology 112: 115-129.
19. Shiroma ME, Damous LL, Cotrim FP, Roa CL, Cipolla-Neto J, Reiter RJ, Baracat EC, Soares JM Jr (2021) Pretreatment with melatonin improves ovarian tissue cryopreservation for transplantation. Reprod. Biol. Endocrinol. 19:17.
20. Plendl J (2000) Angiogenesis and vascular regression in the ovary. Anatomia histologia embryologia 29: 257-266.
21. Shimizu T, Jiang JY, Sasada H, Sato E (2002) Changes of messenger RNA expression of angiogenic factors and related receptors during follicular development in gilts. Biol. Reprod. 67: 1846– 1852.
22. Maganhin CC, Baracat MCP, Carvalho KC, Seganfredo IB, Luquetti CM, Dos Santos Simões R, Carbonel AAF, de Jesus Simões M, Cipolla-Neto J, Girão MJBC, Baracat EC, Soares-Jr JM (2020) Evidence that melatonin increases inhibin beta-a and follistatin gene expression in ovaries of pinealectomized rats. Reprod. Sci. 27:1455-1464.
23. Shiroma ME, Botelho NM, Damous LL, Baracat EC, Soares JM Jr (2016) Melatonin influence in ovary transplantation: systematic review. J. Ovarian. Res. 9:33.
24. Stouffer RL, Martínez-Chequer JC, Molskness TA, Xu F, Hazzard TM (2001) Regulation and action of angiogenic factors in the primate ovary. Arch. Med. Res. 32: 567-575.
25. Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL,Schoolcraft WB (2015) Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil. Steril. 103: 303-316.
26. Ferreira CS, Carvalho KC, Maganhin CC, Paiotti AP, Oshima CT, Simões MJ, Baracat EC, Soares JM Jr (2016) Does melatonin influence the apoptosis in rat uterus of animals exposed to continuous light? Apoptosis 21: 155-162.
27. Lima GN, Maganhin CC, Simões RS, Baracat MC, Sasso GR,Fuchs LF, Simões M DE J, Baracat EC, SOares Júnior JM (2015) Steroidogenesis-related gene expression in the rat ovary exposed to melatonin supplementation. Clinics (Sao Paulo) 70: 144-151.
28. Priya DM, Akhtar N, Ahmad J (2015) Prevalence of hypothyroidism in infertile women and evaluation of response of treatment for hypothyroidism on infertility. Indian. J. Endocrinol. Metab. 19: 504-506.
29. Tal R, Seifer DB, Arici A (2015) The emerging role of angiogenic factor dysregulation in the pathogenesis of polycystic ovarian syndrome Semin. Reprod. Med. 33: 195-207.
30. Fraser HM, Duncan WC (2009). Regulation and manipulation of angiogenesis in the ovary and endometrium. Reprod. Fertil. Dev. 21: 377–392.
31. Myers M, Pangas SA (2010) Regulatory roles of transformin ggrowth factor beta family members in folliculogenesis. Wiley Interdiscip. Rev. Syst. Biol. Med. 2: 117-125.
32. Monniaux D, Clément F, Dalbiès-tran R, Estienne A, Fabre S, Mansanet C, Monget P (2014) The ovarian reserve of primordial follicles and the dynamic reserve of antral growing follicles: what is the link? Biol. Reprod. 90: 85.
33. Chaves RN, de Matos MH, Buratini J Jr, de Figueiredo JR (2012) The fibroblast growth factor family: involvement in the regulation of folliculogenesis. Reprod. Fertil. Dev. 24: 905-915.
34. Baskind NE, Orsi NM, Sharma V (2014) Follicular-phase ovarian follicular fluid and plasma cytokine profiling of natural cycle in vitro fertilization patients. Fertil. Steril. 102: 410-418.
35. Scotti L, Abramovich D, Pascuali N1, Irusta G, Meresman G, Tesone M, Parborell F (2014) Local VEGF inhibition prevents ovarian alterations associated with ovarian hyperstimulation syndrome J. Steroid. Biochem. Mol. Biol. 144 Pt B: 392-401.
36. Park JA, Kim DY, Kim YM, Lee IK, Kwon YG (2015) Endothelial snail regulates capillary branching morphogenesis via vascular endothelial growth factor receptor 3 expression. PLoS Genet.11: e1005324.
37. Duncan WC, Nio-kobayashi J (2013) Targeting angiogenesis in th epathological ovary. Reprod. Fertil. Dev. 25: 362-371.
38. Anhê GF, Caperuto LC, Pereira-Da-Silva M, Souza LC, Hirata AE, Velloso LA, Cipolla-Neto J, Carvalho CR (2004) In vivo activation of insulin receptor tyrosine kinase by melatonin in the rat hypothalamus. J. Neurochem. 90: 559-566.
39. Nishimura R, Okuda K (2020) Multiple roles of hypoxia in bovine corpus luteum. J. Reprod. Dev. 66: 307-310.
40. Gervásio CG, Bernuci MP, Silva-de-sá MF, Rosa-e-silva AC (2014) The role of androgen hormones in early follicular development. ISRN Obstet. Gynecol. 2014: 818010.
41. Fuhrmeister IP, Branchini G, Pimentel AM, Ferreira GD, Capp E, Brum IS, Von eye corleta H (2014) Human granulosa cells: insulin and insulin-like growth factor-1 receptors and aromatase expression modulation by metformin. Gynecol. Obstet. Invest. 77: 156-162.
42. Bach LA (2015) Endothelial cells and the IGF system. J. Mol. Endocrinol. 54: R1-13.
43. Lombardi LA, Mattos LS, Simões RS, Florencio-Silva R, Sasso GRDS, Carbonel AAF, Simões MJ, Baracat EC, Soares JM Jr (2019) Melatonin may prevent or reverse polycystic ovary syndrome in rats. Rev. Assoc. Med. Bras. 65:1008-1014.
44. Tamura I, Tamura H, Kawamoto-Jozaki M, Shirafuta Y, Fujimura T, Doi-Tanaka Y, Mihara Y, Taketani T, Sugino N (2022). Effects of melatonin on the transcriptome of human granulosa cells, fertilization and blastocyst formation. Int. J. Mol. Sci. 23: 6731.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


