Combination of melatonin with paclitaxel reduces the TLR4-mediated inflammatory pathway, PD-L1 levels, and survival of ovarian carcinoma cells
Melatonin and paclitaxel in the OC cell
Abstract
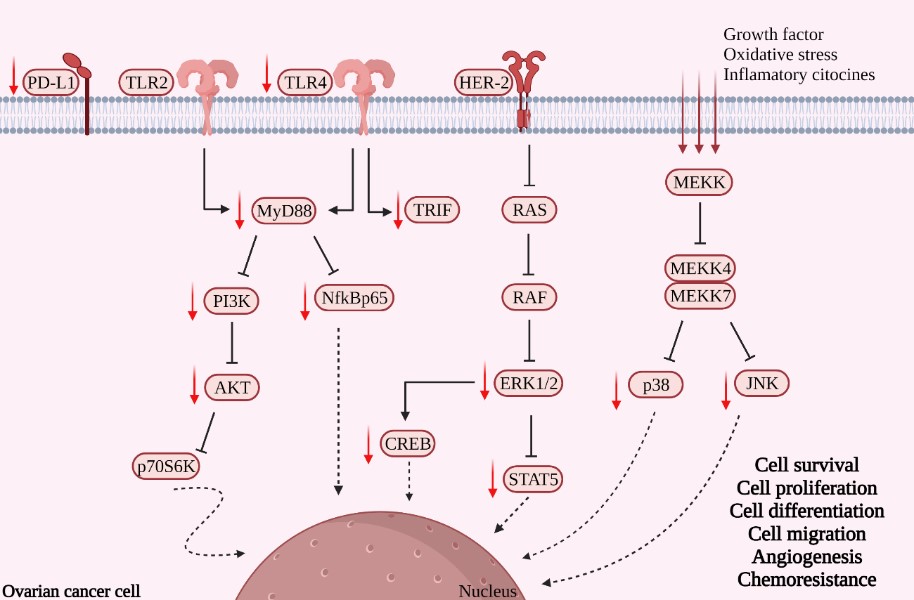
Ovarian cancer (OC) has a high mortality rate. Although most patients respond to the conventional chemotherapy [e.g., paclitaxel (PTX)], some also develop drug resistance to make the treatment less effective. Since melatonin exhibits antioxidant, antitumor, and immunomodulatory functions in a variety of solid tumors, in this study the effects of a combination of PTX and melatonin on SKOV-3 human ovarian carcinoma cells were investigated and the focus was given to the Toll-like receptor (TLR)-mediated inflammatory pathway and cell signaling-related molecules. Flow cytometry showed that this combination significantly boosted the apoptosis/necrosis responses of the cancer cells. Cell migration was attenuated by melatonin alone, and the combination led to a reduced number of migrating and invasive cells. Melatonin alone and its combination also reduced the levels of TLR4, MyD88, TRIF, and PD-L1, but not TLR2. In addition, the combination significantly lowered the levels of NF-kB p65, PI3K, p-AKT, p38, ERK 1/2, JNK, CREB, p70s6K, and STAT5. The results suggested that this combination was effective in reducing the viability and the invasive capacity of SKOV-3 cells while increasing their apoptosis and necrosis rates. The potential mechanism of this combination is to attenuate the downstream molecules of the TLR4-mediated inflammatory pathway and cell signaling-related proteins in the cancer cells. Thus, melatonin improved the chemosensitivity of the cancer cells to PTX, serving as an effective adjuvant therapy against OC.
References
2. Feeney L, Harley IJ, McCluggage WG, Mullan PB, Beirne JP (2020) Liquid biopsy in ovarian cancer: Catching the silent killer before it strikes. World J. Clin. Oncol. 11: 868–889 https://doi.org/10.5306/wjco.v11.i11.868.
3. Jessmon P, Boulanger T, Zhou W, Patwardhan P (2017) Epidemiology and treatment patterns of epithelial ovarian cancer. Expert Rev. Anticancer Ther. 17: 427–437 https://doi.org/10.1080/14737140.2017.1299575.
4. Jayson GC, Kohn EC, Kitchener HC, Ledermann JA (2014) Ovarian cancer. Lancet 384: 1376–1388 https://doi.org/10.1016/S0140-6736(13)62146-7.
5. Stewart C, Ralyea C, Lockwood S (2019) Ovarian cancer: An integrated review. Semin. Oncol. Nurs. 35: 151–156 https://doi.org/10.1016/j.soncn.2019.02.001.
6. Orr B, Edwards RP (2018) Diagnosis and treatment of ovarian cancer. Hematol. Oncol. Clin. North Am. 32: 943–964 https://doi.org/10.1016/j.hoc.2018.07.010.
7. Bernabeu E, Cagel M, Lagomarsino E, Moretton M, Chiappetta DA (2017) Paclitaxel: What has been done and the challenges remain ahead. Int. J. Pharm. 526: 474–495 https://doi.org/10.1016/j.ijpharm.2017.05.016.
8. Fu Y, Li S, Zu Y, Yang G, Yang Z, Luo M, Jiang S, Wink M, Efferth T (2009) Medicinal chemistry of paclitaxel and its analogues. Curr. Med. Chem. 16: 20 https://doi.org/10.2174/092986709789352277.
9. Bast RC, Hennessy B, Mills GB (2009) The biology of ovarian cancer: new opportunities for translation. Nat. Rev. Cancer 9: 415–428 https://doi.org/10.1038/nrc2644.
10. Chuffa LGA, Fioruci-Fontanelli BA, Mendes LO, Ferreira Seiva FR, Martinez M, Fávaro WJ, Domeniconi RF, Pinheiro PF, Delazari dos Santos L, Martinez FE (2015) Melatonin attenuates the TLR4-mediated inflammatory response through MyD88- and TRIF-dependent signaling pathways in an in vivo model of ovarian cancer. BMC Cancer 15: 34 https://doi.org/10.1186/s12885-015-1032-4.
11. Gatti L, Zunino F (2005) “Overview of tumor cell chemoresistance mechanisms” in Chemosensitivity: Volume II: In VIVO Models, Imaging, and Molecular Regulators, Methods in Molecular Medicine, Blumenthal RD, Ed. (Humana Press), pp. 127–148 https://doi.org/10.1385/1-59259-889-7:127.
12. Byrd-Leifer CA, Block EF, Takeda K, Akira S, Ding A (2001) The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol. Eur. J. Immunol. 31: 2448–2457 https://doi.org/10.1002/1521-4141(200108)31:8<2448::aid-immu2448>3.0.co;2-n.
13. Wang A-C, Ma Y-B, Wu F-X, Ma Z-F, Liu N-F, Gao R, Gao Y-S, Sheng X-G (2014) TLR4 induces tumor growth and inhibits paclitaxel activity in MyD88-positive human ovarian carcinoma in vitro. Oncol. Lett. 7: 871–877 https://doi.org/10.3892/ol.2013.1759.
14. Kelly MG, Alvero AB, Chen R, Silasi D-A, Abrahams VM, Chan S, Visintin I, Rutherford T, Mor G (2006) TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 66: 3859–3868 https://doi.org/10.1158/0008-5472.CAN-05-3948.
15. Huang B, Zhao J, Li H, He K-L, Chen Y, Chen S-H, Mayer L, Unkeless JC, Xiong H (2005) Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 65: 5009–5014 https://doi.org/10.1158/0008-5472.CAN-05-0784.
16. Huang B, Zhao J, Unkeless JC, Feng ZH, Xiong H (2008) TLR signaling by tumor and immune cells: a double-edged sword. Oncogene 27: 218–224 https://doi.org/10.1038/sj.onc.1210904.
17. Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, Edwards MR, Michelsen KS, Kroeger KM, Liu C, et al. (2009) HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 6: e10 https://doi.org/10.1371/journal.pmed.1000010.
18. Oliva J, Bardag-Gorce F, Li J, French BA, French SW (2011) S-adenosylmethionine prevents the up regulation of Toll-like receptor (TLR) signaling caused by chronic ethanol feeding in rats. Exp. Mol. Pathol. 90: 239–243 https://doi.org/10.1016/j.yexmp.2011.01.005.
19. Taniguchi K, Karin M (2018) NF-κB, inflammation, immunity and cancer: coming of age. Nat. Rev. Immunol. 18: 309–324 https://doi.org/10.1038/nri.2017.142.
20. Yu J, Wang X, Teng F, Kong L (2016) PD-L1 expression in human cancers and its association with clinical outcomes. OncoTargets Ther. 9: 5023–5039 https://doi.org/10.2147/OTT.S105862.
21. Wakabayashi G, Lee Y-C, Luh F, Kuo C-N, Chang W-C, Yen Y (2019) Development and clinical applications of cancer immunotherapy against PD-1 signaling pathway. J. Biomed. Sci. 26: 96 https://doi.org/10.1186/s12929-019-0588-8.
22. Bailey A, McDermott DF (2013) Immune checkpoint inhibitors as novel targets for renal cell carcinoma therapeutics. Cancer J. 19: 348–352 https://doi.org/10.1097/PPO.0b013e31829e3153.
23. Kim JY, Cho CH, Song HS (2017) Targeted therapy of ovarian cancer including immune check point inhibitor. Korean J. Intern. Med. 32: 798–804 https://doi.org/10.3904/kjim.2017.008.
24. Howlader N, Noone A, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis D, et al. (2018) SEER Cancer Statistics Review, 1975-2018. Based Novemb. 2018 SEER Data Submiss. Posted SEER Web Site April 2019 (September 13, 2021) https://seer.cancer.gov/csr/1975_2016/index.html.
25. Yuan TL, Cantley LC (2008) PI3K pathway alterations in cancer: variations on a theme. Oncogene 27: 5497–5510 https://doi.org/10.1038/onc.2008.245.
26. Meric Bernstam F, Hung M-C (2006) Advances in targeting human epidermal growth factor receptor-2 signaling for cancer therapy. Clin. Cancer Res. 12: 6326–6330 https://doi.org/10.1158/1078-0432.CCR-06-1732.
27. Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103: 239–252 https://doi.org/10.1016/s0092-8674(00)00116-1.
28. Johnson GL, Lapadat R (2002) Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298: 1911–1912 https://doi.org/10.1126/science.1072682.
29. Achkar IW, Abdulrahman N, Al-Sulaiti H, Joseph JM, Uddin S, Mraiche F (2018) Cisplatin based therapy: the role of the mitogen activated protein kinase signaling pathway. J. Transl. Med. 16: 96 https://doi.org/10.1186/s12967-018-1471-1.
30. Dhanasekaran DN, Reddy EP (2008) JNK signaling in apoptosis. Oncogene 27: 6245–6251 https://doi.org/10.1038/onc.2008.301.
31. Huang C, Zhou L, Chang X, Pang X, Zhang H, Zhang S (2016) B7-H3, B7-H4, Foxp3 and IL-2 expression in cervical cancer: Associations with patient outcome and clinical significance. Oncol. Rep. 35: 2183–2190 https://doi.org/10.3892/or.2016.4607.
32. Jiang XL, Gao JC, Jiang L, Zhang PX, Kang TJ, Sun Q, Qi WJ, Zhang QP, Guan HW, Shi H (2019) Expression and significance of MAPK/ERK in the specimens and cells of epithelial ovarian cancer]. Zhonghua Fu Chan Ke Za Zhi 54: 541–547 https://doi.org/10.3760/cma.j.issn.0529-567x.2019.08.007.
33. Levy DE, Darnell JE (2002) Stats: transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 3: 651–662 https://doi.org/10.1038/nrm909.
34. Yu H, Pardoll D, Jove R (2009) STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer 9: 798–809 https://doi.org/10.1038/nrc2734.
35. Wu C-J, Sundararajan V, Sheu B-C, Huang RY-J, Wei L-H (2019) Activation of STAT3 and STAT5 signaling in epithelial ovarian cancer progression: Mechanism and therapeutic opportunity. Cancers 12: 24 https://doi.org/10.3390/cancers12010024.
36. Chen M-W, Yang S-T, Chien M-H, Hua K-T, Wu C-J, Hsiao SM, Lin H, Hsiao M, Su J-L, Wei L-H (2017) The STAT3-miRNA-92-Wnt signaling pathway regulates spheroid formation and malignant progression in ovarian cancer. Cancer Res. 77: 1955–1967 https://doi.org/10.1158/0008-5472.CAN-16-1115.
37. Jinawath N, Vasoontara C, Jinawath A, Fang X, Zhao K, Yap K-L, Guo T, Lee CS, Wang W, Balgley BM, et al. (2010) Oncoproteomic analysis reveals co-upregulation of RELA and STAT5 in carboplatin resistant ovarian carcinoma. PLOS ONE 5: e11198 https://doi.org/10.1371/journal.pone.0011198.
38. Sauer LA, Dauchy RT, Blask DE (2001) Polyunsaturated fatty acids, melatonin, and cancer prevention. Biochem. Pharmacol. 61: 8 https://doi.org/10.1016/s0006-2952(01)00634-7.
39. Reiter R, Rosales-Corral S, Tan D-X, Acuna-Castroviejo D, Qin L, Yang S-F, Xu K (2017) Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. Int. J. Mol. Sci. 18: 843 https://doi.org/10.3390/ijms18040843.
40. Reiter RJ (2004) Melatonin relieves the neural oxidative burden that contributes to dementias. Ann. N. Y. Acad. Sci. 1035: 179–196 https://doi.org/10.1196/annals.1332.012.
41. Chuffa LGA, Fioruci-Fontanelli BA, Mendes LO, Fávaro WJ, Pinheiro PFF, Martinez M, Martinez FE (2013) Characterization of chemically induced ovarian carcinomas in an ethanol-preferring rat model: influence of long-term melatonin treatment. PLoS ONE 8: e81676 https://doi.org/10.1371/journal.pone.0081676.
42. Chuffa LGA, Lupi Júnior LA, Seiva FRF, Martinez M, Domeniconi RF, Pinheiro PFF, dos Santos LD, Martinez FE (2016) Quantitative proteomic profiling reveals that diverse metabolic pathways are influenced by melatonin in an in vivo model of ovarian carcinoma. J. Proteome Res. 15: 3872–3882 https://doi.org/10.1021/acs.jproteome.6b00713.
43. Ferreira GM, Martinez M, Camargo ICC, Domeniconi RF, Martinez FE, Chuffa LGA (2014) Melatonin attenuates Her-2, p38 MAPK, p-AKT, and mTOR levels in ovarian carcinoma of ethanol-preferring rats. J. Cancer 5: 728–735.
44. Agarwal R, Kaye SB (2003) Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat. Rev. Cancer 3: 502–516 https://doi.org/10.1038/nrc1123.
45. Wu X, Zhao J, Ruan Y, Sun L, Xu C, Jiang H (2018) Sialyltransferase ST3GAL1 promotes cell migration, invasion, and TGF-β1-induced EMT and confers paclitaxel resistance in ovarian cancer. Cell Death Dis. 9: 1102 https://doi.org/10.1038/s41419-018-1101-0.
46. Conti A, Conconi S, Hertens E, Skwarlo-Sonta K, Markowska M, Maestroni GJM (2000) Evidence for melatonin synthesis in mouse and human bone marrow cells. J. Pineal Res. 28: 193–202 https://doi.org/10.1034/j.1600-079X.2000.280401.x.
47. Zhang J, Xie T, Zhong X, Jiang H-L, Li R, Wang B-Y, Huang X-T, Cen B-H, Yuan Y-W (2020) Melatonin reverses nasopharyngeal carcinoma cisplatin chemoresistance by inhibiting the Wnt/β-catenin signaling pathway. Aging 12: 5423–5438 https://doi.org/10.18632/aging.102968.
48. Fernandez-Gil BI, Guerra-Librero A, Shen Y-Q, Florido J, Martínez-Ruiz L, García-López S, Adan C, Rodríguez-Santana C, Acuña-Castroviejo D, Quiñones-Hinojosa A, et al. (2019) Melatonin enhances cisplatin and radiation cytotoxicity in head and neck squamous cell carcinoma by stimulating mitochondrial ros generation, apoptosis, and autophagy. Oxid. Med. Cell. Longev. 2019: e7187128 https://doi.org/10.1155/2019/7187128.
49. Sakatani A, Sonohara F, Goel A (2019) Melatonin-mediated downregulation of thymidylate synthase as a novel mechanism for overcoming 5-fluorouracil associated chemoresistance in colorectal cancer cells. Carcinogenesis 40: 422–431 https://doi.org/10.1093/carcin/bgy186.
50. Chuffa LG de A, Reiter RJ, Lupi LA (2017) Melatonin as a promising agent to treat ovarian cancer: molecular mechanisms. Carcinogenesis 38: 945–952 https://doi.org/10.1093/carcin/bgx054.
51. Akbarzadeh M, Nouri M, Banekohal MV, Cheraghi O, Tajalli H, Movassaghpour A, Soltani S, Cheraghi H, Feizy N, Montazersaheb S, et al. (2016) Effects of combination of melatonin and laser irradiation on ovarian cancer cells and endothelial lineage viability. Lasers Med. Sci. 31: 1565–1572 https://doi.org/10.1007/s10103-016-2016-6.
52. Akbarzadeh M, Movassaghpour AA, Ghanbari H, Kheirandish M, Fathi Maroufi N, Rahbarghazi R, Nouri M, Samadi N (2017) The potential therapeutic effect of melatonin on human ovarian cancer by inhibition of invasion and migration of cancer stem cells. Sci. Rep. 7: 17062 https://doi.org/10.1038/s41598-017-16940-y.
53. Kim KH, Jo MS, Suh DS, Yoon MS, Shin DH, Lee JH, Choi KU (2012) Expression and significance of the TLR4/MyD88 signaling pathway in ovarian epithelial cancers. World J. Surg. Oncol. 10: 193 https://doi.org/10.1186/1477-7819-10-193.
54. Ding Z, Wu X, Wang Y, Ji S, Zhang W, Kang J, Li J, Fei G (2020) Melatonin prevents LPS-induced epithelial-mesenchymal transition in human alveolar epithelial cells via the GSK-3β/Nrf2 pathway. Biomed. Pharmacother. 132: 110827 https://doi.org/10.1016/j.biopha.2020.110827.
55. Ammar OA, El-Missiry MA, Othman AI, Amer ME (2022) Melatonin is a potential oncostatic agent to inhibit HepG2 cell proliferation through multiple pathways. Heliyon 8: e08837 https://doi.org/10.1016/j.heliyon.2022.e08837.
56. Lupi LA, Delella FK, Cucielo MS, Romagnoli GG, Kaneno R, Nunes I da S, Domeniconi RF, Martinez M, Martinez FE, Fávaro WJ, et al. (2019) P-MAPA and interleukin-12 reduce cell migration/invasion and attenuate the Toll-Like receptor-mediated inflammatory response in ovarian cancer SKOV-3 cells: A preliminary study. Molecules 25: 5 https://doi.org/10.3390/molecules25010005.
57. Bu S, Wang Q, Sun J, Li X, Gu T, Lai D (2020) Melatonin suppresses chronic restraint stress-mediated metastasis of epithelial ovarian cancer via NE/AKT/β-catenin/SLUG axis. Cell Death Dis. 11: 644 https://doi.org/10.1038/s41419-020-02906-y.
58. Li Y, Li S, Zhou Y, Meng X, Zhang J-J, Xu D-P, Li H-B (2017) Melatonin for the prevention and treatment of cancer. Oncotarget 8: 39896–39921 https://doi.org/10.18632/oncotarget.16379.
59. Wang J, Xiao X, Zhang Y, Shi D, Chen W, Fu L, Liu L, Xie F, Kang T, Huang W, et al. (2012) Simultaneous modulation of COX-2, p300, Akt, and Apaf-1 signaling by melatonin to inhibit proliferation and induce apoptosis in breast cancer cells: Melatonin modulates COX-2, p300, Akt, and Apaf-1. J. Pineal Res. 53: 77–90 https://doi.org/10.1111/j.1600-079X.2012.00973.x.
60. Casado-Zapico S, Rodriguez-Blanco J, Garcia-Santos G, Martin V, Sanchez-Sanchez AM, Antolin I, Rodriguez C (2010) Synergistic antitumor effect of melatonin with several chemotherapeutic drugs on human Ewing sarcoma cancer cells: potentiation of the extrinsic apoptotic pathway. J. Pineal Res. 48: 72–80 https://doi.org/10.1111/j.1600-079X.2009.00727.x.
61. Gao Y, Xiao X, Zhang C, Yu W, Guo W, Zhang Z, Li Z, Feng X, Hao J, Zhang K, et al. (2017) Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing PI3K/AKT and NF-κB/iNOS signaling pathways. J. Pineal Res. 62: e12380 https://doi.org/10.1111/jpi.12380.
62. Zhao M, Wan J, Zeng K, Tong M, Lee AC, Ding J, Chen Q (2016) The Reduction in circulating melatonin level may contribute to the pathogenesis of ovarian cancer: A retrospective study. J. Cancer 7: 831–836 https://doi.org/10.7150/jca.14573.
63. Altaf MA, Shahid R, Ren M-X, Mora-Poblete F, Arnao MB, Naz S, Anwar M, Altaf MM, Shahid S, Shakoor A, et al. (2021) Phytomelatonin: An overview of the importance and mediating functions of melatonin against environmental stresses. Physiol. Plant 172: 820–846 https://doi.org/10.1111/ppl.13262.
64. Gata V, Laurentiu IF (2017) The role of Toll-like receptors in ovarian cancer. J. BUON 22: 1092–1096.
65. Huang X, Hou X, Chuan L, Wei S, Wang J, Yang X, Ru J (2020) miR-129-5p alleviates LPS-induced acute kidney injury via targeting HMGB1/TLRs/NF-kappaB pathway. Int. Immunopharmacol. 89: 107016 https://doi.org/10.1016/j.intimp.2020.107016.
66. Zhou M, McFarland-Mancini MM, Funk HM, Husseinzadeh N, Mounajjed T, Drew AF (2009) Toll-like receptor expression in normal ovary and ovarian tumors. Cancer Immunol. Immunother. CII 58: 1375–1385 https://doi.org/10.1007/s00262-008-0650-y.
67. Vlad C, Dina C, Kubelac P, Vlad D, Pop B, Achimas Cadariu P (2018) Expression of toll-like receptors in ovarian cancer. J. BUON Off. J. Balk. Union Oncol. 23: 1725–1731.
68. Li W, Wu J, Li Z, Zhou Z, Zheng C, Lin L, Tan B, Huang M, Fan M (2016) Melatonin induces cell apoptosis in Mia PaCa-2 cells via the suppression of nuclear factor-κB and activation of ERK and JNK: A novel therapeutic implication for pancreatic cancer. Oncol. Rep. 36: 2861–2867 https://doi.org/10.3892/or.2016.5100.
69. Wang AC, Su QB, Wu FX, Zhang XL, Liu PS (2009) Role of TLR4 for paclitaxel chemotherapy in human epithelial ovarian cancer cells. Eur. J. Clin. Invest. 39: 157–164 https://doi.org/10.1111/j.1365-2362.2008.02070.x.
70. Perera P-Y, Mayadas TN, Takeuchi O, Akira S, Zaks-Zilberman M, Goyert SM, Vogel SN (2001) CD11b/CD18 acts in concert with CD14 and Toll-Like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J. Immunol. 166: 574–581 https://doi.org/10.4049/jimmunol.166.1.574.
71. Rajput S, Volk-Draper LD, Ran S (2013) TLR4 is a novel determinant of the response to paclitaxel in breast cancer. Mol. Cancer Ther. 12: 1676–1687 https://doi.org/10.1158/1535-7163.MCT-12-1019.
72. Sun N-K, Huang S-L, Chang T-C, Chao CC-K (2018) TLR4 and NFκB signaling is critical for taxol resistance in ovarian carcinoma cells. J. Cell. Physiol. 233: 2489–2501 https://doi.org/10.1002/jcp.26125.
73. Lau TS, Chan LKY, Man GCW, Wong CH, Lee JHS, Yim SF, Cheung TH, McNeish IA, Kwong J (2020) Paclitaxel induces immunogenic cell death in ovarian cancer via TLR4/IKK2/SNARE-dependent exocytosis. Cancer Immunol. Res. 8: 1099–1111 https://doi.org/10.1158/2326-6066.CIR-19-0616.
74. Yan X-Y, Qu X-Z, Xu L, Yu S-H, Tian R, Zhong X-R, Sun L-K, Su J (2020) Insight into the role of p62 in the cisplatin resistant mechanisms of ovarian cancer. Cancer Cell Int. 20: 128 https://doi.org/10.1186/s12935-020-01196-w.
75. Yilmaz E, Gul M, Melekoglu R, Koleli I (2018) Immunhistochemical analysis of nuclear factor kappa beta expression in etiopathogenesis of ovarian tumors. Acta Cir. Bras. 33: 641–650 https://doi.org/10.1590/s0102-865020180070000009.
76. Colombo J, Perassi-Jardim BV, Ferreira JPS, Braga CZ, Sonehara NM, Júnior RP, Moshchetta MG, Girol AP, Zuccari DAPC (2018) Melatonin differentially modulates NF-кB expression in breast and liver cancer cells. Anticancer Agents Med. Chem. 18: 1688–1694. https://doi.org/10.2174/1871520618666180131112304.
77. Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, Baba T, Konishi I, Mandai M (2015) IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br. J. Cancer 112: 1501–1509 https://doi.org/10.1038/bjc.2015.101.
78. Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T (2013) A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat. Immunol. 14: 1212–1218 https://doi.org/10.1038/ni.2762.
79. Nhokaew W, Kleebkaow P, Chaisuriya N, Kietpeerakool C (2019) Programmed death ligand 1 (PD-L1) expression in epithelial ovarian cancer: A comparison of type I and type II tumors. Asian Pac. J. Cancer Prev. 20: 1161–1169 https://doi.org/10.31557/APJCP.2019.20.4.1161.
80. Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, Iyer AK (2017) PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front. Pharmacol. 8: 561 https://doi.org/10.3389/fphar.2017.00561.
81. Sharma P, Allison JP (2015) The future of immune checkpoint therapy. Science 348: 56–61 https://doi.org/10.1126/science.aaa8172.
82. Chang M-C, Chen C-A, Chen P-J, Chiang Y-C, Chen Y-L, Mao T-L, Lin H-W, Lin Chiang W-H, Cheng W-F (2012) Mesothelin enhances invasion of ovarian cancer by inducing MMP-7 through MAPK/ERK and JNK pathways. Biochem. J. 442: 293–302 https://doi.org/10.1042/BJ20110282.
83. Echevarría-Vargas IM, Valiyeva F, Vivas-Mejía PE (2014) Upregulation of miR-21 in cisplatin resistant ovarian cancer via JNK-1/c-Jun pathway. PLOS ONE 9: e97094 https://doi.org/10.1371/journal.pone.0097094.
84. Bildik G, Akin N, Senbabaoglu F, Esmalian Y, Sahin GN, Urman D, Karahuseyinoglu S, Ince U, Palaoglu E, Taskiran C, et al. (2018) Endogenous c-Jun N-terminal kinase (JNK) activity marks the boundary between normal and malignant granulosa cells. Cell Death Dis. 9: 421 https://doi.org/10.1038/s41419-018-0459-3.
85. Steven A, Friedrich M, Jank P, Heimer N, Budczies J, Denkert C, Seliger B (2020) What turns CREB on? And off? And why does it matter? Cell. Mol. Life Sci. 77: 4049–4067 https://doi.org/10.1007/s00018-020-03525-8.
86. Yu H, Jove R (2004) The STATs of cancer — new molecular targets come of age. Nat. Rev. Cancer 4: 97–105 https://doi.org/10.1038/nrc1275.
87. Min H, Wei-hong Z (2009) Constitutive activation of signal transducer and activator of transcription 3 in epithelial ovarian carcinoma. J. Obstet. Gynaecol. Res. 35: 918–925 https://doi.org/10.1111/j.1447-0756.2009.01045.x.
88. Gasparri M, Bardhi E, Ruscito I, Papadia A, Farooqi A, Marchetti C, Bogani G, Ceccacci I, Mueller M, Benedetti Panici P (2017) PI3K/AKT/mTOR pathway in ovarian cancer treatment: Are we on the right track? Geburtshilfe Frauenheilkd. 77: 1095–1103 https://doi.org/10.1055/s-0043-118907.
89. Cesário RC, Gaiotte LB, Cucielo MS, Silveira HS, Delazari dos Santos L, de Campos Zuccari DAP, Seiva FRF, Reiter RJ, de Almeida Chuffa LG (2022) The proteomic landscape of ovarian cancer cells in response to melatonin. Life Sci. 294: 120352 https://doi.org/10.1016/j.lfs.2022.120352.
90. Chuffa LG, Fioruci-Fontanelli B, Mendes L, Pinheiro P, Martinez M, Martinez F (2014) TLR4-mediated signaling pathway is modulated by melatonin through MyD88-dependent pathway in ovarian carcinoma of ethanol-consuming rats. Reprod. Abstr. 1: P122 https://doi.org/10.1530/repabs.1.P122.
91. Lin Y-W, Lee L-M, Lee W-J, Chu C-Y, Tan P, Yang Y-C, Chen W-Y, Yang S-F, Hsiao M, Chien M-H (2016) Melatonin inhibits MMP-9 transactivation and renal cell carcinoma metastasis by suppressing Akt-MAPKs pathway and NF- κ B DNA-binding activity. J. Pineal Res. 60: 277–290 https://doi.org/10.1111/jpi.12308.
92. Zare H, Shafabakhsh R, Reiter RJ, Asemi Z (2019) Melatonin is a potential inhibitor of ovarian cancer: molecular aspects. J. Ovarian Res. 12: 26 https://doi.org/10.1186/s13048-019-0502-8.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


