Pineal melatonin deprivation alters the mRNA expression of melatonin and steroidogenic-related receptor genes in rat oviduct and uterus during the estrus stage of estrous cycle
Melatonin and steroidogenic-related receptors in uterus
Abstract
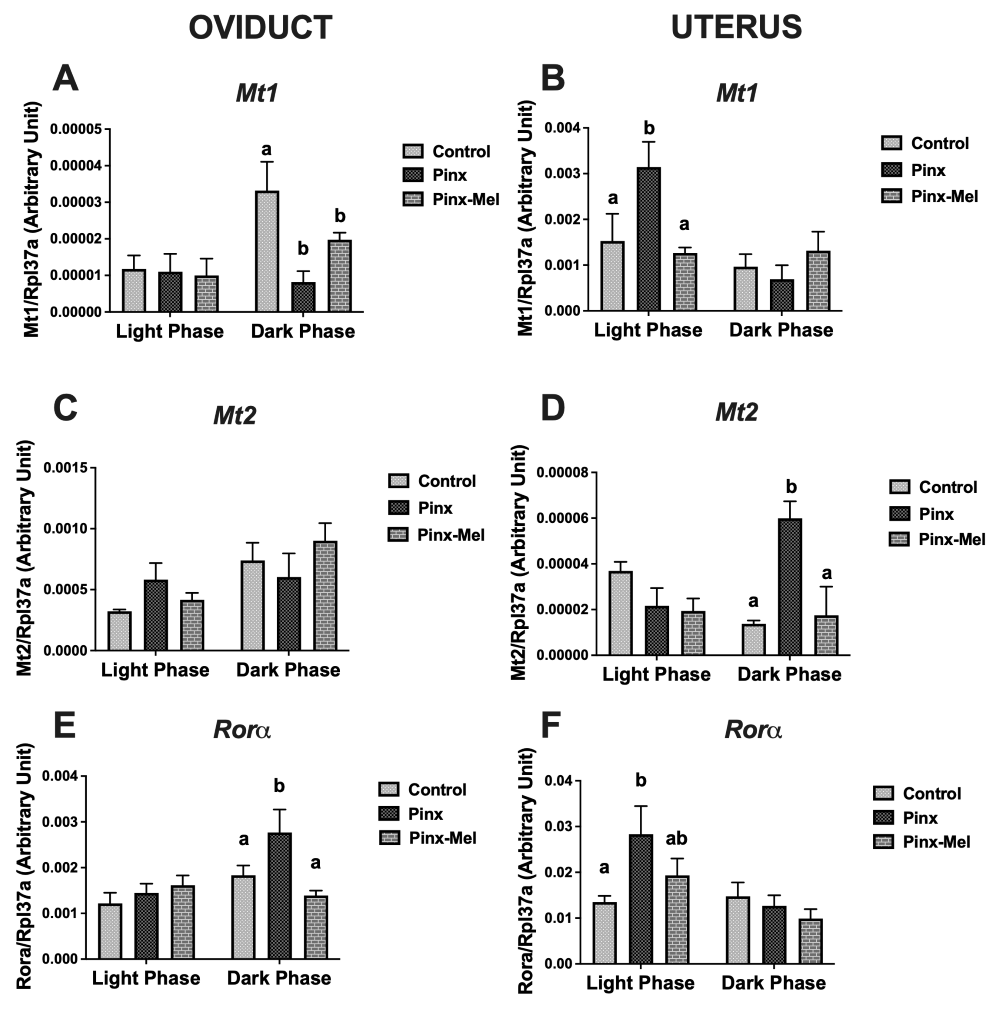
The effects of pinealectomy and melatonin replacement therapy on the mRNA expression of melatonin (Mt1, Mt2 and Rora), steroidogenic (Era - Estrogen a, Erb - Estrogen b, Ar - Androgen, Pgr - Progesterone, Lhr - LH and Oxr - Oxytocin receptors) -related receptors and melatonin-synthetic enzyme genes (Tph1, Asmt and Aanat) in oviduct and uterus of Wistar rats were studied. Tissues were collected from sham-pinealectomized (Control), pinealectomized (Pinx) and Pinx plus melatonin- supplemented (Pinx-Mel) females during the estrus stage of both the light (ZT6) and dark (ZT18) phases of the light-dark cycle. In oviduct, Pinx altered the mRNA expression of Mt1, Mt2, Rora, Era, Pgr, Oxr, Asmt and Aanat genes. In uterus, Pinx altered the mRNA expression of Mt1, Mt2, Rora, Lhr, Pgr, Oxr and Tph1 genes. Melatonin treatment partially or completely restored the Mt2, Rora, Era, Oxr, Asmt and Aanat mRNA expressions in oviduct and the Mt1, Mt2 and Rora mRNA expressions in uterus. These alterations varied according to the phase of light-dark cycle. The results suggest that pineal melatonin regulates the daily mRNA expression of melatonin and steroidogenic-related receptors in the rat oviduct and uterus during the estrus stage of estrous cycle. Melatonin also regulates the daily mRNA expression of Asmt and Aanat in oviduct and Tph1 in uterus.
References
2. Senger PL (2005) Pathways to Pregnancy and Parturition, 2nd ed. (Current Conceptions Inc., Washington, USA), pp 154-283.
3. Gallo RV, Babu GN, Bona-Gallo A, Devorshak-Harvey RE, Leipheimer RE, Marco J (1987) Regulation of pulsatile luteinizing hormone release during the estrous cycle and pregnancy in the rat. Adv. Exp. Med. Biol. 219: 109-130.
4. An S-M, Kim SS, Kim J, Park M-N, Lee J-E, Cho SK, Lee K-S, An SK (2017) Expression of reproductive hormone receptors and contraction-associated genes in porcine uterus during the estrous cycle. Mol. Med. Rep. 15: 4176-4184.
5. Critchley HOD, Henderson TA, Kelly RW, Scobie GS, Evans LR, Groome NP, Saunders PTK (2002) Wild-type estrogen receptor (ER1) and the slice variant (ERcx/2) are both expressed within the human endometrium throughout the normal menstrual cycle. J. Clin. Endocrinol. Metab. 11: 5265-5273.
6. Chuffa LGA, Seiva FRF, Fávaro WJ, Teixeira GR, Amorim JPA, Mendes LO, Fioruci BA, Pinheiro PFF, Fernandes AAH, Franci JAA, Dellela FK, Martinez M, Martinez FE (2011) Melatonin reduces LH, 17 beta-estradiol and induces differential regulation of sex steroid receptors in reproductive tissues during rat ovulation. Reprod. Biol. Endocrinol. 9: 108.
7. Tamura H, Nakamura Y, Korkmaz A, Manchester LC, Tan D-X, Sugino N, Reiter RJ (2009) Melatonin and the ovary: physiological and pathophysiological implications. Fertil. Steril. 92: 328-343.
8. Reiter RJ, Tamura H, Tan D-X, Xu X-Y (2014) Melatonin and the circadian system: contributions to successful female reproduction. Fertil. Steril. 102: 321-328.
9. Reiter RJ, Tan D-X, Korkmaz A, Rosales-Corral AS (2014) Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum. Reprod. Update. 20: 293-307.
10. Bahadori MH, Ghasemian F, Ramezani M, Asgari Z (2013) Melatonin effect during different maturation stages of oocyte and subsequent embryo development in mice. Iran J. Reprod. Med. 11: 11-18.
11. Wei D, Zhang C, Xie J, Song X, Yin B, Liu Q, Hu L, Hao H, Geng J, Wang P (2013) Supplementation with low concentrations of melatonin improves nuclear maturation of human oocytes in vitro. J. Assist. Reprod. Genet. 30: 933-938.
12. Kim MK, Park EA, Kim HJ, Choi WY, Cho JH, Lee WS, Cha KY, Kim YS, Lee DR, Yoon TK (2013) Does supplementation of in-vitro culture medium with melatonin improve IVF outcome in PCOS? Reprod. Biomed. Online 26: 22-29.
13. Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, Taketani T, Matsuoka A, Yamagata Y, Shimamura K, Morioka H, Ishikawa H, Reiter RJ, Sugino N (2008) Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal Res. 44: 280-287.
14. Tamura H, Jozaki M, Tanabe M, Shirafuta Y, Mihara Y, Shinagawa M, Tamura I, Maekawa R, sato S, Taketani T, Takasaki A, Reiter RJ, Sugino N (2020) Importance of melatonin in assisted reproductive technology and ovarian age. Int. J. Mol. Sci. 21: 1135. doi:10.3390/ijms21031135.
15. Chiba A, Akema T, Toyoda J (1994) Effects of pinealectomy and melatonin on the timing of proestrous luteinizing hormone surge in the rat. Neuroendocrinology 59: 163-168.
16. Cagnacci A, Soldani R, Yen SSC (1995) Exogenous melatonin enhances luteinizing hormone levels of women in the follicular but not in the luteal menstrual phase. Fertil. Steril. 68: 996-999.
17. Schlabritz-Loutsevitch N, Hellner N, Middendorf R, Muller D, Olcese J (2003) The human myometrium as a target for melatonin. J. Clin. Endocrinol. Metab. 88: 908-913.
18. Woo MMM, Tai C-J, Kang SK, Nathwani PS, Pang SF, Leung PCK (2011) Direct Action of Melatonin in Human Granulosa-Luteal Cells. J. Clin. Endocrinol. Metab. 2086: 4789-4797.
19. He C, Wang J, Zhang Z, Yang M, Li Y, Tian X, Ma T, Tao J, Zhu K, Song Y, Ji P, Liu G (2016) Mitochondria synthesize melatonin to ameliorate its function and improve mice oocyte’s quality under in vitro conditions. Int. J. Mol. Sci. 17: 939. doi:10.3390/ijms17060939.
20. Coelho LA, Peres R, Amaral FG, Reiter RJ, Cipolla-Neto J (2015) Daily differential expression of melatonin-related genes and clock genes in rat cumulus-oocyte complex: Changes after pinealectomy. J. Pineal Res. 58: 490-499.
21. Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan D-X, Reiter RJ (2014) Extrapineal melatonin: sources, regulation, and potential functions. Cell. Mol. Life Sci. 71: 2997-3025.
22. Coelho LA, Buonfiglio DC, Kuwabara WMT, Andrade-Silva J, Gomes PRL, Scialfa JH. Peres R, Amaral FG, Cipolla-Neto J (2020) Melatonin regulates the expression of Bone Morphogenetic Protein 15 (Bmp-15), Growth Differentiation Factor 9 (Gdf-9) and LH receptor (Lhr) genes in developing follicles of rats. Melatonin Res. 3: 515-533, doi: 10.32794/mr11250076.
23. He C, Wang J, Li Y, Zhu K, Xu Z, Song Y, Song Y, Liu G (2015) Melatonin-related genes expressed in the mouse uterus during early gestation promote embryo implantation. J. Pineal Res. 58: 300-309.
24. Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L, Tamura I, Maekawa R, Asada H, Yamagata Y, Sugino N (2012) The role of melatonin as an antioxidant in the follicle. J. Ovarian Res. 5: 5. doi:10.1186/1757-2215-5-5.
25. Reiter RJ, Tan D-X, Terron MP, Flores LJ, Czarnocki Z (2007) Melatonin and its metabolites: new findings regarding their production and their radical scavenging actions. Acta Biochim. Pol. 54: 1-9.
26. Soares Jr JM, Masana MI, Ersahinn Ç, Dubocovich ML (2003) Funcional melatonin receptors in rat ovaries at various stages of estrous cycle. J. Pharmacol. Exp. Ther. 306: 694-702.
27. Seithikurippu R, Pandi-Perumal R, Trakht I, Srinivasan V, Spence DW, Maestroni GJM, Zisapel N, Cardinali DP (2008) Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 85: 335-353.
28. Becker-André M, Wiesenberg I, Schaeren-Wiemers N, André E, Missbach, M, Saurat J-H, Carlberg C (1994) Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J. Biol. Chem. 269: 28531-28534.
29. Coelho LA, Andrade-Silva J, Motta-Teixeira LC, Amaral FG, Reiter RJ, Cipolla-Neto J (2019) The absence of pineal melatonin abolishes the daily rhythm of Tph1 (Tryptophan Hydroxylase 1), Asmt (Acetylserotonin O-Methyltransferase) and Aanat (Aralkylamine N- Acetyltransferase) mRNA expressions in rat testes. Mol. Neurobiol. 56: 7800-7809.
30. Paccola, CC, Resende CG, Stumpp T, Miraglia SM, Cipriano I (2013) The rat cycle revisited: a quantitative and qualitative analysis. Anim. Reprod. 10: 677-683.
31. Hoffman RA, Reiter RJ (1965) Rapid pinealectomy in hamsters and other small rodents. Anat. Rec. 153: 19-21.
32. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25: 402-408.
33. Talpur HS, Worku T, Rehman Z, Dad R, Bhattarai D, Bano I, Farmanullah, Liang A, He C, Yang L (2017) Knockdown of melatonin receptor 1 and induction of follicle-stimulating hormone on the regulation of mouse granulosa cell function. Reprod. Biol. 17: 380-388.
34. Riaz H, Yousuf MR, Liang A, Hua GH, Yang L (2019) Effect of melatonin on regulation of apoptosis and steroidogenesis in culture buffalo granulosa cells. Anim. Sci. J. 90: 473-480.
35. Tian X, Wang F, Zhang L, He C, Ji P, Wang J, Zhang Z, Lv D, Abulizi W, Wang X, Lian Z, Liu G (2017) Beneficial effects of melatonin on the in vitro maturation of sheep oocytes and its relation to melatonin receptors. Int. J. Mol. Sci. 18: 834, doi:10.3390/ijms18040834.
36. He C, Ma T, Shi J, Zhang Z, Wang J, Zhu K, Li Y, Yang M, Song Y, Liu G (2016) Melatonin and its receptor MT1 are involved in the downstream reaction to luteinizing hormone and participate in the regulation of luteinization in the different species. J. Pineal Res. 61: 279-290.
37. Wang S-J, Liu W-J, Wu C-J, Ma F-H, Ahmad S, Liu B-R, Han L, Jiang X-P, Zhang S-J, Yang L-G (2012) Melatonin suppresses apoptosis and stimulates progesterone production by bovine granulosa cells via its receptors (Mt1 and Mt2). Theriogenology 78: 1517-1526.
38. Wang S-J, Liu W-J, Wang L-K, Pang X-S, Yang, L-G (2017) The role of melatonin receptor MTNR1A in the action of melatonin on bovine granulosa cells. Mol. Reprod. Dev. 84: 1140-1154.
39. Sanghoon L, Jun-Xue J, Anukul T, Geon-A K, Byeong-Chun L (2018) Stimulatory Effects of Melatonin on Porcine In Vitro Maturation Are Mediated by MT2 Receptor. Int. J. Mol. Sci. 19: 1581. doi:10.3390/ijms19061581.
40. Wang S, Liu W, Pang X, Dai S, Liu G (2018) The mechanism of melatonin and its receptor MT2 involved in the development of bovine granulosa cells. Int. J. Mol. Sci. 19: 2028. doi:10.3390/ijms19072028.
41. Zhao H, Pang S.F, Poon AMS (2002) mt1 Receptor-mediated antiproliferative effects of melatonin on the rat uterine antimesometrial stromal cells. Mol. Reprod. Dev. 61: 192-199.
42. Venegas C, García JÁ, Doerrier HV, Volt H, Escames G, López LS, Reiter RJ, Acuña-Castroviejo D (2013) Analysis of the daily changes of melatonin receptors in the rat liver. J. Pineal Res. 54: 313-321.
43. Nakamura TJ, Sellix MT, Kudo T, Nakao N, Yoshimura T, Ebihara S, Colwell C.S, Block GD (2010) Influence of the estrous cycle on clock gene expression in reproductive tissues: Effects of fluctuating ovarian steroid hormones levels. Steroids 75: 203-212.
44. Bivin WS, Crawford MP, Brewer NR (1979) Effects of endocrine secretions on estrus. Biology of the rat, eds Baker HJ, Lindsey JR, Weisbroth SH (Academic Press, New York, USA), pp. 92-93.
45. Soares JM, Simões MJ, Oshima CTF, Mora OA, Lima GR, Baracat EC (2003) Pinealectomy changes rat ovarian interstitial cell morphology and decreases progesterone receptor expression. Gynecol. Endocrinol. 17: 115-123.
46. Dair EL, Simões RS, Simões MJ, Romeu LRG, Oliveira-Filho RM, Haidar MA, Baracat EC, Soares JM Jr (2008) Effects of melatonin on the endometrial morphology and embryo implantation in rats. Fertil. Steril. 89: 1299-1305.
47. Hu J-J, Xiao L-F, Song L-L, Ge W-B, Duan H-W, Jiang Y (2020) The expression of melatonin receptors MT1 and MT2 is regulated by E2 in sheep oviduct. Gen. Comp. Endocrinol. 286: 113135. Doi.org/10.1016/j.ygcen.2019.03.004.
48. Zhao H, Pang S.F, Poon AMS (2002) Variations of mt1 melatonin receptor density in the rat uterus during decidualization, the estrous cycle and in response to exogenous steroid treatment. J. Pineal. Res. 33: 140-145.
49. Li S, O’Neill SRS, Zhang Y, Holtzman MJ, Takemaru, K-I, Korach KS, Winuthayanon W (2017) Estrogen receptor is required for oviductal transport of embryos. FASEB J. 31: 1595-1607.
50. An SM, Kim SS, Kim J, Park MN, Lee JE, Cho SK, Lee KS, An BS (2017) Expression of reproductive hormone receptors and contraction-associated genes in porcine uterus during the estrous cycle. Mol. Med. Rep. 15: 4176-4184.
51. Lecce G, Meduri G, Ancelin M, Bergeron C, Perrot-Applanat M (2001) Presence of estrogen receptor in the human endometrium through the cycle: expression in glandular, stromal, and vascular cells. J. Clin. Endocrinol. Metab. 86: 1379-1386.
52. Wang H, Eriksson H, Sahlin L (2000) Estrogen receptors and in the reproductive tract of the rat during estrous cycle. Bio. Reprod. 63: 1331-1340.
53. Ulbrich SE, Kettler A, Einspanier R (2003) Expression and localization of estrogen receptor , estrogen receptor and progesterone receptor in the bovine oviduct in vivo and in vitro. J. Steroid Biochem. Mol. Biol. 84: 279-289.
54. Ayad VJ, McGoff SA, Wathes DC (1990) Oxytocin receptors in the oviduct during the oestrous cycle of the ewe. J. Endocrinol. 124: 353-359.
55. Larcher A, Neculcea J, Breton C, Arslan A, Rozen F, Russo C, Zingg HH (1995) Oxytocin receptor gene expression in the rat uterus during pregnancy and estrous cycle and in response to gonadal steroid treatment. Endocrinology 136: 5350-5356.
56. Gimeno MF, Landa A, Sterin-Speziale N, Cardinali DP, Gimeno AL (1980) Melatonin blocks in vitro generation of prostaglandin by the uterus and hypothalamus. Eur. J. Pharmacol. 62: 309-317.
57. Roberts JS, McCracken JA, Gavagan JE, Soloff MS Oxytocin-stimulated release of prostaglandin F2 from ovine endometrium in vitro: correlation with estrous cycle and oxytocin-receptor binding. Endocrinology 99: 1107-1114.
58. Ayar A, Kutlu S, Yilmaz B, Kelestimur H (2001) Melatonin inhibits spontaneous and oxytocin-induced contractions of rat myometrium in vitro. Neuro Endocrinol. Lett. 22: 199-207.
59. Kasahara Y, Kitahara Y, Nakamura K, Minegishi T (2012) Downregulation of LH receptor mRNA in rat uterus. Mol. Med. Rep. 25: 1146-1150.
60. Lin DX, Lei ZM, Li X, Ch, Rao V (2005) Targeted disruption of LH receptor gene revealed the importance of uterine LH signaling. Mol. Cell. Endocrinol. 234: 105-116.
61. Yoriki K, Mori T, Kokabu T, Matsushima H, Umemura S, Tarumi Y, Kitawaki J (2019) Estrogen-related receptor alpha induces epithelial-mesenchymal transition through cancer-stromal interactions in endometrial cancer. Sci. Rep. 9: 6697. doi.org/10.1038/s41598-019-43261-z.
62. Liu A, Zhang D, Yang X, Song Y (2019) Estrogen receptor alpha activates MAPK signaling pathway to promote the development of endometrial cancer. J. Cell. Biochem. 120 (10):17593-17601. Doi: 10.1002/jcb.29027.
63. Hu G, Zhang J, Zhou X, Liu J, Wang Q, Zhang B (2020) Roles of estrogen receptor and in the regulation of proliferation in endometrial carcinoma. Pathol. Res. Pract. 216 (10):153149. doi.org/10.1016/j.prp.2020.153149.
64. Christofoloni DM, Vilarino FL, Mafra FA, André GM, Bianco B, Barbosa CP (2011) Combination of polymorphisms in luteinizing hormone , estrogen receptor and progesterone receptor and susceptibility to infertility and endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 158: 260-264.
65. de Carvalho CV, Nogueira-de-Souza NC, Costa AMM, Baracat EC, Girão MJBC, D’Amora P, Schor E, da Silva IDCG (2007) Genetic polymorphisms of cytochrome P450c17 (CYP17) and progesterone receptor genes (PROGINS) in the assessment of endometriosis risk. Gynecol. Endocrinol. 23: 29-33.
66. Gu C, Yan, H, Chang K, Zhang B, Xie F, Ye J, Chang R, Qiu X, Wang Y, Qu Y, Wang J, Li M (2020) Melatonin alleviates progression of uterine endometrial cancer by suppressing estrogen/ubiquitin C/SDHB-mediated succinate accumulation. Cancer Lett. 476: 34-47.
67. Watanabe M, Kobayashi Y, Takahashi N, Kiguchi K, Ishizuda B (2008) Expression of melatonin receptor (MT1) and interaction between melatonin and estrogen in endometrial cancer cell line. J. Obstet. Gynecol. Res. 4: 567-573.
68. Qi S, Yan L, Liu Z, Mu Y-I, Li M, Zhao X, Chen Z-J (2018) Melatonin inhibits 17-estradiol-induced migration, invasion, and epithelial-mesenchymal transition in normal and endometriotic endometrial epithelial cells. Reprod. Biol. Endocrinol. 16: 62. doi.org/10.1186/s12958-018-0375-5.
69. Yang H-L, Zhou W-J, Gu C-J, Meng Y-H, Shao J, Li D-J, Li M-Q (2018) Pleiotropic roles of melatonin in endometriosis, recurrent spontaneous abortion, and polycystic ovary syndrome. Am. J. Reprod. Immunol. 80: e12839. DOI: 10.1111/aji.12839.
70. Cipolla-Neto J, Amaral FG (2018) Melatonin as a hormone: new physiological and clinical insights. Endocr. Rev. 39: 990-1028.
71. Gomes PRL, Vilas-Boas EA, Leite EA, Munhoz AC, Lucena CF, Amaral FG, Carpinelli AR, Cipolla-Neto J (2021) Melatonin regulates maternal pancreatic remodeling and B-cell function during pregnancy and lactation. J. Pineal Res. 71: e12717. doi: 10.1111/jpi.12717.
72. Amaral FG, Andrade-Silva J, Kuwabara WMT, Cipolla-Neto J (2019) New insights into the function of melatonin and its role in metabolic disturbances. Expert Rev. Endocrinol. Metab. 14: 293-300.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


