Melatonin synthesized by activated microglia orchestrates the progression of microglia from a pro-inflammatory to a recovery/repair phenotype
Melatonin and cerebellar microglia phenotypes
Abstract
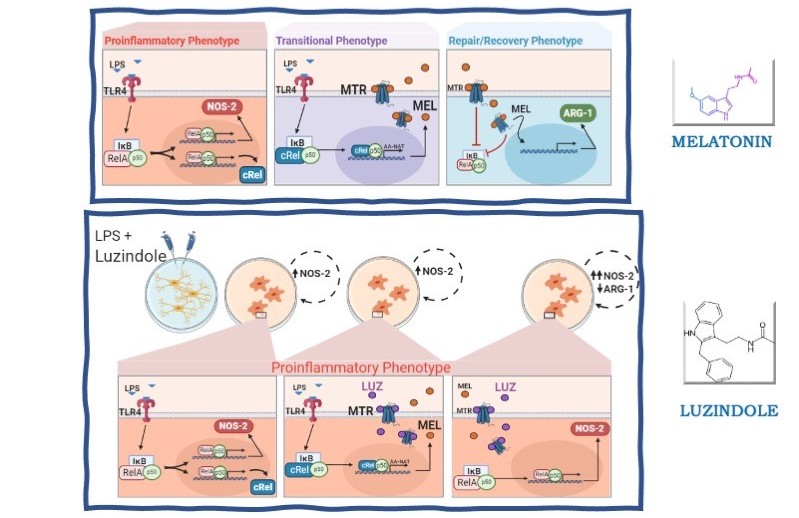
Microglia, the sentinels of the central nervous system, are responsible for the surveillance and the innate defense against pathogen or danger/damage-associated molecular patterns. The response is fine-tuned to restrain pro-inflammatory responses, preserving neighboring cells. At the injured area, microglia temporarily shift to a pro-inflammatory phenotype (M1), followed by anti-inflammatory (M2) phenotypes. The duration and magnitude of the pro-inflammatory phase are finely regulated to avoid unnecessary loss of brain tissue. The present study shows that melatonin synthesized by microglia plays a key role in the transformation of M1 to M2 phenotypes. In a mixed rat cerebellar glia culture, the percentage of activated microglia did not vary significantly with the treatments, while the role of melatonin synthesized by microglia in promoting the end of the pro-inflammatory phase, and the initiation of the regulatory/phagocytic phases was inferred by using pharmacological tools. Total microglia were identified by the expression of CD11b/c, whereas positive to IBA-1 microglia were considered activated, independent of the phenotype. M1 and M2 phenotypes were distinguished with the biomarkers NOS-2 and ARG-1, as these enzymes act on the same substrate (L-arginine), producing pro-inflammatory (NO) or anti-inflammatory (polyamines and proline) end products, respectively. Luzindole, a blocker of melatonin receptors, impaired the conversion of M1 to M2 phenotypes and zymosan phagocytosis. Thus, melatonin content synthesized by cerebellar microglia determines the extension of the pro-inflammatory phase of defense response.
References
2. Stence N, Waite M, Dailey ME (2001). Dynamics of microglial activation: A confocal time-lapse analysis in hippocampal slices. Glia 33 (3): 256–266.
3. Kaltschmidt B, Kaltschmidt C (2010) NF-kappaB in the nervous system. Cold Spring Harb Perspect Biol. 1 (3):a001271. doi: 20066105.
4. Sun SC (2017). The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 17 (9), 545-558.
5. Thawkar BS, Kaur G (2019). Inhibitors of NF-κB and P2X7/NLRP3/Caspase 1 pathway in microglia: Novel therapeutic opportunities in neuroinflammation induced early-stage Alzheimer's disease. J. Neuroimmunol. 326: 62-74.
6. Jie Z, Ko CJ, Wang H, Xie X, Li Y, Gu M, Zhu L, Yang JY, Gao T, Ru W, Tang SJ, Cheng X, Sun SC (2021) Microglia promote autoimmune inflammation via the noncanonical NF-κB pathway. Sci Adv. 7 (36): eabh0609. https://doi.org/10.1126/sciadv.abh0609
7. De Jesús TJ, Ramakrishnan P (2020). NF-κB c-Rel dictates the inflammatory threshold by acting as a transcriptional repressor. iScience 23 (3): 100876, https://doi.org/10.1016/j.isci.2020.100876.
8. Muxel SM, Laranjeira-Silva MF, Carvalho CE, Floeter-Winter LM, Markus RP (2016). The RelA/cRel nuclear factor-ĸB ( NF- ĸB ) dimer, crucial for inflammation resolution, mediates the transcription of the key enzyme in melatonin synthesis in RAW 264.7 macrophages. J Pineal Res. 60 (4): 394–404.
9. Carvalho-Sousa CE, Pereira EP, Kinker GS, Veras M, Ferreira ZS, Barbosa-Nunes FP, Markus RP (2020). Immune-pineal axis protects rat lungs exposed to polluted air. J. Pineal Res. 68 (3): 1–13.
10. Markus RP, Cecon E, Pires-Lapa MA (2013) Immune-pineal axis: Nuclear factor κB (NF-κB) mediates the shift in the melatonin source from pinealocytes to immune competent cells. Int. J. Mol. Sci. 14 (6): 10979–10997.
11. Pires-Lapa MA, Tamura K, Salustiano EMA, Markus RP (2013) Melatonin synthesis in human colostrum mononuclear cells enhances dectin-1-mediated phagocytosis by mononuclear cells. J. Pineal Res. 55 (3): 240–246.
12. GaoJ, Su G, Liu J, Zhang J, Zhou J, Liu X, Zhang Z (2020). Mechanisms of inhibition of excessive microglial activation by melatonin. J. Mol. Neurosci. 7 (8): 1229–1236.
13. Liu W, Yu M, Xie D, Wang L, Ye C, Zhu Q, Yang L (2020) Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res. Ther. 11 (1): 1–15.
14. Markus RP, Fernandes PA, Kinker GS, da Silveira Cruz-Machado S, Marçola M (2018) Immune-pineal axis – acute inflammatory responses coordinate melatonin synthesis by pinealocytes and phagocytes. Bri. J. Pharmacol. 175 (16): 3239–3250.
15. Mecha M (2011) An easy and fast way to obtain a high number of glial cells from rat cerebral tissue: A beginners approach. Protocol Exchange 218: 1–18.
16. Tamashiro TT, Dalgard CL, Byrnes KR (2012) Primary microglia isolation from mixed glial cell cultures of neonatal rat brain tissue. J. Vis. Exp. 66: e3814, 1–5. https://doi.org/10.3791/3814.
17. Jockers R, Delagrange P, Dubocovich ML, Zlotos DP (2016) Update on melatonin receptors: IUPHAR Review 20. Bri. J. Pharmacol. 173: 2702-2725.
18. Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, Kadomatsu K (2013) Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 4 (3): e525-9. https://doi.org/10.1038/cddis.2013.54.
19. Nikodemova M, Duncan ID, Watters JJ (2006) Minocycline exerts inhibitory effects on multiple mitogen-activated protein kinases and IκBα degradation in a stimulus-specific manner in microglia. J. Neurochem. 96 (2): 314–323.
20. Franco DG, Markus RP (2014) The cellular state determines the effect of melatonin on the survival of mixed cerebellar cell culture. PLOS One 9 (9): 25–27.
21. Kaltschmidt B, Widera D, Kaltschmidt C (2005) Signaling via NF-κB in the nervous system. Biochim. Biophys. Acta. 1745 (3): 287–299.
22. Muxel SM, Pires-Lapa MA, Monteiro AWA, Cecon E, Tamura EK, Floeter-Winter L M, Markus RP (2012) NF-κB drives the synthesis of melatonin in RAW 264.7 macrophages by inducing the transcription of the arylalkylamine-N-acetyltransferase (AA-NAT) gene. PLOS ONE 7 (12): e52010. https://doi.org/10.1371/journal.pone.0052010.
23. Bernier LP, Bohlen CJ, York EM, Choi HB, Kamyabi A, Dissing-Olesen L, MacVicar BA (2019) Nanoscale surveillance of the brain by microglia via cAMP-regulated filopodia. Cell Rep. 27 (10): 2895-2908.e4. https://doi.org/10.1016/j.celrep.2019.05.010.
24. Wu UI, Mai FD, Sheu JN, Chen LY, Liu YT, Huang HC, Chang HM. (2011) Melatonin inhibits microglial activation, reduces pro-inflammatory cytokine levels, and rescues hippocampal neurons of adult rats with acute Klebsiella pneumoniae meningitis. J. Pineal Res. 50 (2) ; 159–170.
25. Berkiks I, Benmhammed H, Mesfioui A, Ouichou A, El Hasnaoui A, Mouden S El Hessni A (2018) Postnatal melatonin treatment protects against affective disorders induced by early-life immune stimulation by reducing the microglia cell activation and oxidative stress. Int. J. Neurosci. 128 (6): 495–504.
26. Hu L, Zhang S, Wen H, Liu T, Cai J, Du D, Xia C (2019) Melatonin decreases M1 polarization via attenuating mitochondrial oxidative damage depending on UCP2 pathway in prorenin-treated microglia. PLOS ONE. 14 (2): 1–18.
27. Park E, Chun H S (2017) Melatonin attenuates manganese and lipopolysaccharide-induced inflammatory activation of BV2 microglia. Neurochem. Res. 42 (2): 656–666.
28. Zhang Y, Liu Z, Zhang W, Wu Q, Zhang Y, Liu, Y, Chen X (2019) Melatonin improves functional recovery in female rats after acute spinal cord injury by modulating polarization of spinal microglial/macrophages. J. Neurosci. Res. 97 (7): 733–743.
29. Leung JWH, Cheung TK (2021) Melatonin attenuates microglial activity and omproves neurological functions in rat model of collagense-induced intracerebral hemorrage. Melatonin Res. 4 (2): 360-376.
30. Pontes GN, Cardoso EC, Carneiro-Sampaio MMS, Markus RP (2006) Injury switches melatonin production source from endocrine (pineal) to paracrine (phagocytes) - Melatonin in human colostrum and colostrum phagocytes. J. Pineal Res. 41 (2): 136–141.
31. Pinato L, da Silveira Cruz-Machado S, Franco DG, Campos LMG, Cecon E, Fernandes PACM, Markus RP (2015) Selective protection of the cerebellum against intracerebroventricular LPS is mediated by local melatonin synthesis. Brain Struct. Funct. 220 (2): 827–840.
32. Ohsawa K, Imai Y, Sasaki Y, Kohsaka S (2004) Microglia/macrophage-specific protein Iba1 binds to fimbrin and enhances its actin-bundling activity. J. Neurochem. 88 (4): 844–856.
33. Chhor V, Le Charpentier T, Lebon S, Oré MV, Celador IL, Josserand J, Fleiss B (2013) Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain Behav. Immun. 32: 70–85.
34. Hoogland ICM, Westhoff D, Engelen-Lee JY, Melief J, Valls Serón M, Houben-Weerts JHMP, van de Beek D (2018) Microglial activation after systemic stimulation with lipopolysaccharide and Escherichia coli. Front. Cell. Neurosci. 12: 110.
35. Cherry JD, Olschowka JA, Banion MK O (2014) Neuroinflammation and M2 microglia : the good , the bad , and the inflamed, J Neuroinflammation. 11 (1): 1–15.
36. Lee M, Rey K, Besler K, Wang C, Choy J (2017) Immunobiology of nitric oxide and regulation of inducible nitric oxide synthase. Results Probl. Cell. Differ. 62: 181–207.
37. Caldwell RW, Rodriguez PC, Toque HA, Narayanan SP, Caldwell RB (2018) Arginase: A multifaceted enzyme important in health and disease. Physiol. Rev. 98 (2): 641–665.
38. Hamzei Taj S, Kho W, Riou A, Wiedermann D, Hoehn M (2016) miRNA-124 induces neuroprotection and functional improvement after focal cerebral ischemia. Biomaterials 91: 151–165.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


