Accuracy and precision of 31P-MRS assessment for evaluating the effect of melatonin-pretreated mitochondria transferring on liver fibrosis of rats
Quantitative 31P-MRS for detecting hepatic fibrosis
Abstract
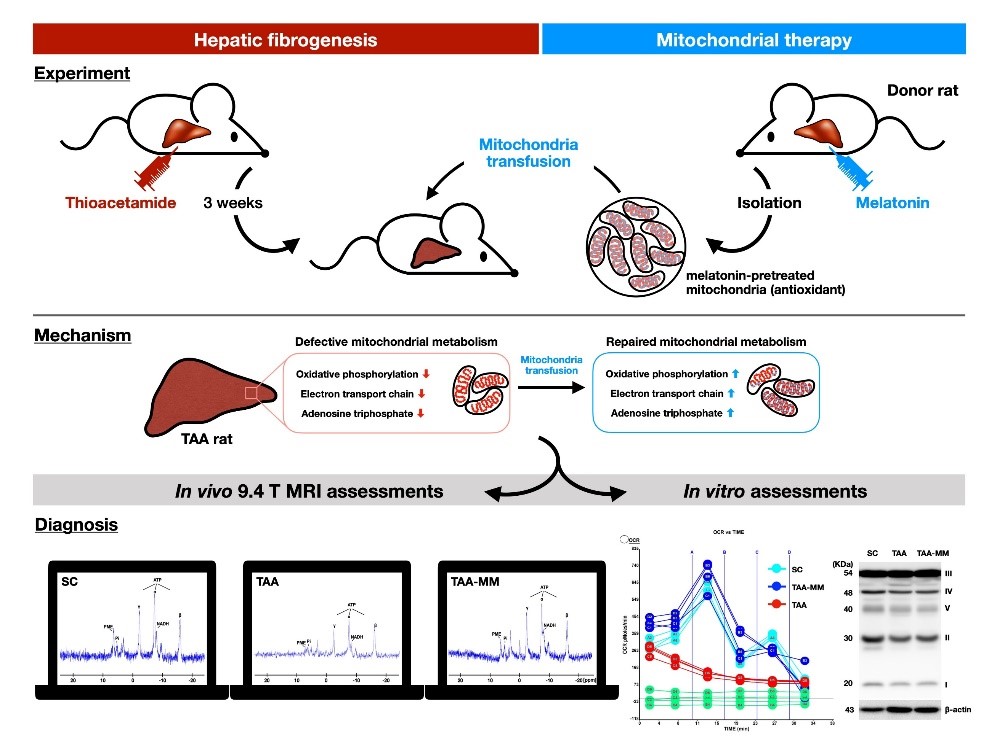
This study examined the reliability of 31phosphorus-magnetic resonance spectroscopy (31P-MRS) to measure parameters of liver metabolic function in the intact animals. These parameters can help us to evaluate the severity and prognosis of liver fibrosis. In addition, 31P-MRS was also used to examine the protective effects of melatonin on liver mitochondria. An animal model of liver fibrosis was established via intraperitoneal administration of thioacetamide (TAA) to rats. Rats were scanned at baseline, week 3 and 6 after TAA treatment, respectively, to measure the longitudinal changes of phosphorus metabolite levels by 31P-MRS at 9.4 T. The results showed a consistent decline in the levels of phosphorus metabolites (inorganic phosphate, α-ATP, γ-ATP and NADH) in rats with fibrosis. Impaired mitochondrial respiration capacity, collagen accumulation and the extent of fibrosis in liver were markedly associated with decreased concentrations of phosphorus metabolites. Melatonin-pretreated mitochondria transferring efficiently prevented TAA-induced liver damage mainly by restoring mitochondrial function. In conclusion, the levels of phosphorus metabolites could serve as the indicators of mitochondrial oxidative capacity and thus provides a novel tool to evaluate mitochondrial integrity in the in vivo condition by using 31P-MRS in the setting of liver fibrosis.
References
2. Bataller R, Brenner DA (2005) Liver fibrosis. J. Clin. Invest. 115 (2): 209-218.
3. Abdel-Misih SR, Bloomston M (2010) Liver anatomy. Surg. Clin. North Am. 90 (4): 643-653.
4. Schmeltzer PA, Talwalkar JA (2011) Noninvasive tools to assess hepatic fibrosis: ready for prime time? Gastroenterol. Clin. North Am. 40 (3): 507-521.
5. Bedossa P, Paradis V (2003) Liver extracellular matrix in health and disease. J. Pathol. 200 (4): 504-515.
6. Bedossa P, Carrat F (2009) Liver biopsy: the best, not the gold standard. J. Hepatol. 50 (1): 1-3.
7. Janes CH, Lindor KD (1993) Outcome of patients hospitalized for complications after outpatient liver biopsy. Ann. Intern Med. 118 (2): 96-98.
8. Cardi M, et al. (1997) Superiority of laparoscopy compared to ultrasonography in diagnosis of widespread liver diseases. Dig. Dis. Sci. 42 (3): 546-548.
9. Li S, et al. (2019) Liver fibrosis conventional and molecular imaging diagnosis update. J. Liver. 8 (1): 236.
10. Kudo M, et al. (2008) Diagnostic accuracy of imaging for liver cirrhosis compared to histologically proven liver cirrhosis. A multicenter collaborative study. Intervirology 51 (Suppl 1): 17-26.
11. Yeom SK, Lee CH, Cha SH, Park CM (2015) Prediction of liver cirrhosis, using diagnostic imaging tools. World J. Hepatol. 7 (17): 2069-2079.
12. Samuel D, Feray C, Bismuth H (1997) Hepatitis viruses and liver transplantation. J. Gastroenterol. Hepatol. 12 (9-10): S335-341.
13. Prompers JJ, et al. (2006) Dynamic MRS and MRI of skeletal muscle function and biomechanics. NMR Biomed. 19 (7): 927-953.
14. Valkovic L, Chmelik M, Krssak M (2017) In-vivo (31)P-MRS of skeletal muscle and liver: A way for non-invasive assessment of their metabolism. Anal. Biochem. 529: 193-215.
15. Liu Y, Gu Y, Yu X (2017) Assessing tissue metabolism by phosphorous-31 magnetic resonance spectroscopy and imaging: a methodology review. Quant. Imaging Med. Surg. 7 (6): 707-726.
16. Corbin IR, et al. (2003) Hepatic 31P MRS in rat models of chronic liver disease: assessing the extent and progression of disease. Gut 52 (7): 1046-1053.
17. Degli Esposti D et al. (2012) Mitochondrial roles and cytoprotection in chronic liver injury. Biochem. Res. Int. 2012: 387626.
18. Lane M, et al. (2016) Mitochondrial dysfunction in liver failure requiring transplantation. J. Inherit. Metab. Dis. 39 (3): 427-436.
19. Rahman S (2013) Gastrointestinal and hepatic manifestations of mitochondrial disorders. J. Inherit. Metab. Dis. 36 (4): 659-673.
20. Reiter RJ, et al. (2003) Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 50 (4): 1129-1146.
21. Sun CK, et al. (2015) Systemic combined melatonin-mitochondria treatment improves acute respiratory distress syndrome in the rat. J. Pineal Res. 58 (2): 137-150.
22. Chen HH, et al. (2016) Melatonin pretreatment enhances the therapeutic effects of exogenous mitochondria against hepatic ischemia-reperfusion injury in rats through suppression of mitochondrial permeability transition. J. Pineal Res. 61 (1): 52-68.
23. Ko SF, et al. (2020) Hepatic (31) P-magnetic resonance spectroscopy identified the impact of melatonin-pretreated mitochondria in acute liver ischaemia-reperfusion injury. J. Cell Mol. Med. 24 (17):10088-10099.
24. Sun CK, et al. (2017) Melatonin treatment enhances therapeutic effects of exosomes against acute liver ischemia-reperfusion injury. Am. J. Transl. Res. 9 (4): 1543-1560.
25. Tahan V, et al. (2004) Melatonin reduces dimethylnitrosamine-induced liver fibrosis in rats. J. Pineal Res. 37 (2): 78-84.
26. Yang HY et al. (2019) Dendropanax morbifera ameliorates thioacetamide-induced hepatic fibrosis via TGF-beta1/Smads pathways. Int. J. Biol. Sci. 15 (4): 800-811.
27. Reif S, et al. (2004) Treatment of thioacetamide-induced liver cirrhosis by the Ras antagonist, farnesylthiosalicylic acid. J. Hepatol. 41 (2): 235-241.
28. Wallace MC, et al. (2015) Standard operating procedures in experimental liver research: thioacetamide model in mice and rats. Lab. Anim. 49 (1 Suppl): 21-29.
29. Delire B, Starkel P, Leclercq I (2015) Animal models for fibrotic liver diseases: What we have, what we need, and what is under development. J. Clin. Transl. Hepatol. 3 (1): 53-66.
30. Salguero Palacios R et al. (2008) Activation of hepatic stellate cells is associated with cytokine expression in thioacetamide-induced hepatic fibrosis in mice. Lab. Invest. 88 (11): 1192-1203.
31. Amin A, Mahmoud-Ghoneim D (2011) Texture analysis of liver fibrosis microscopic images: a study on the effect of biomarkers. Acta Biochim. Biophys. Sin. 43 (3): 193-203.
32. Sathesh Kumar S, Ravi Kumar B, Krishna Mohan G (2009) Hepatoprotective effect of Trichosanthes cucumerina Var cucumerina L. on carbon tetrachloride induced liver damage in rats. J. Ethnopharmacol. 123 (2): 347-350.
33. Chen TM, Subeq YM, Lee RP, Chiou TW, Hsu BG (2008) Single dose intravenous thioacetamide administration as a model of acute liver damage in rats. Int. J. Exp. Pathol. 89 (4): 223-231.
34. Jeantet AY, Truchet M, Naulleau G, Martoja R (1991) Effects of bromadiolone on some organs and tissues (liver, kidney, spleen, blood) of coypu (Myocastor coypus). C. R. Acad. Sci. III. 312 (4): 149-156.
35. Bargellini I, et al. (2014) Radiological diagnosis of hepatocellular carcinoma. J. Hepatocell Carcinoma 1: 137-148.
36. Schraml C, et al. (2015) Imaging of HCC-current state of the art. Diagnostics (Basel). 5 (4): 513-545.
37. Ariff B, et al. (2009) Imaging of liver cancer. World J. Gastroenterol. 15 (11): 1289-1300.
38. Reiter RJ, et al. (2016) Melatonin as an antioxidant: under promises but over delivers. J. Pineal Res. 61 (3): 253-278.
39. Reiter RJ, et al. (2017) Melatonin as a mitochondria-targeted antioxidant: one of evolution's best ideas. Cell Mol. Life Sci. 74 (21): 3863-3881.
40. Tan DX, Reiter RJ (2020) An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J. Exp. Bot. 71 (16): 4677-4689.
41. Fu Z, et al. (2020) Cardioprotective Role of melatonin in acute myocardial infarction. Front. Physiol. 11: 366.
42. Tan DX, Manchester LC, Qin L, Reiter RJ (2016) Melatonin: A Mitochondrial Targeting Molecule Involving Mitochondrial Protection and Dynamics. Int. J. Mol. Sci. 17 (12): 2124.
43. Zhao D, et al. (2019) Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. (Lausanne). 10: 249.
44. Ma Z, et al. (2017) Melatonin and mitochondrial function during ischemia/reperfusion injury. Cell Mol. Life Sci. 74 (21): 3989-3998.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


