The bacteriostatic property of melatonin targets peptic ulcer disease and cholangiocarcinoma
Melatonin, peptic ulcer disease and cholangiocarcinoma
Abstract
The marked drop in the frequency of Helicobacter pylori infection resulting from the use of antibiotics and potent anti-acid medications has substantially lowered the prevalence of peptic ulcer disease in recent decades. Management of this condition, however, is challenging because of the escalating perils of antibiotic resistance and the abuse of anti-inflammatory drugs. For example, the increased prevalence of cholangiocarcinomas may associate with this peptic ulcer disease management including the prolonged use of proton pump inhibitors. Cholangiocarcinoma is one of the most lethal cancers and accounts for almost 15% of all hepatic malignancies. This review provides a concise summary of the latest findings in the pathogenetic mechanisms of cholangiocarcinoma, essentially focusing on peptic ulcer disease and its associated therapies. We also suggest interventions that may reduce Helicobacter pylori infection and peptic ulcers with the bacteriostatic agent, melatonin. Melatonin treatment may reduce the incidence of this devastating cancer or improve the outcome of individuals that develop this disease.
References
2. Gustavsson S, Nyren O (1989) Time trends in peptic ulcer surgery, 1956 to 1986. A nation-wide survey in Sweden. Ann. Surg. 210 (6): 704–709. DOI: 10.1097/00000658-198912000-00003.
3. Sachs G (1997) Proton pump inhibitors and acid-related diseases. Pharmacother. J. Hum. Pharmacol. Drug Ther. 17 (1): 22–37. DOI: 10.1002/j.1875-9114.1997.tb03675.x.
4. Sonnenberg A, Everhart JE (1996) The prevalence of self-reported peptic ulcer in the United States. Am. J. Public Health 86 (2): 200–205. DOI: 10.2105/AJPH.86.2.200.
5. Malfertheiner P, Kandulski A, Venerito M (2017) Proton-pump inhibitors: understanding the complications and risks. Nat. Rev. Gastroenterol. Hepatol. 14 (12): 697–710. DOI: 10.1038/nrgastro.2017.117.
6. Segura-López FK, Güitrón-Cantú A, Torres J (2015) Association between Helicobacter spp. infections and hepatobiliary malignancies: A review. World J. Gastroenterol. 21 (5): 1414–1423. DOI: 10.3748/wjg.v21.i5.1414.
7. Peng YC, Lin CL, Hsu WY, Chow WK, Lee SW, Yeh HZ, Chen CC, Kao CH (2018) Association between cholangiocarcinoma and proton pump inhibitors use: A nested case-control study. Front. Pharmacol. 9 (JUL). DOI: 10.3389/fphar.2018.00718.
8. Lanas A, Chan FKL (2017) Peptic ulcer disease. Lancet 390 (10094): 613–624. DOI: 10.1016/S0140-6736(16)32404-7.
9. Lau JY, Sung J, Hill C, Henderson C, Howden CW, Metz DC (2011) Systematic review of the epidemiology of complicated peptic ulcer disease: incidence, recurrence, risk factors and mortality. Digestion 84 (2):102-13. DOI: 10.1159/000323958.
10. Datta DD and Roychowdhury S (2015) To be or not to be: The host genetic factor and beyond in Helicobacter pylori mediated gastro-duodenal diseases. World J. Gastroenterol. 21 (10): 2883–2895. DOI: 10.3748/WJG.V21.I10.2883.
11. Shiotani A, Y Graham D (2002) Pathogenesis and therapy of gastric and duodenal ulcer disease. Med. Clin. North Am. 86 (6): 1447–1466. DOI: 10.1016/S0025-7125(02)00083-4.
12. Huang JQ, Sridhar S, Hunt RH (2002) Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: A meta-analysis. Lancet 359 (9300): 14–22. DOI: 10.1016/S0140-6736(02)07273-2.
13. Lichtenberger LM, Wang ZM, Romero JJ, Ulloa C, Perez JC, Giraud MN, Barreto JC (1995) Non-steroidal anti-inflammatory drugs (NSAIDs) associate with zwitterionic phospholipids: insight into the mechanism and reversal of NSAID-induced gastrointestinal injury. Nat. Med. 1 (2): 154–158. DOI: 10.1038/NM0295-154.
14. Wallace JL (2008) Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn’t the stomach digest itself? Physiol. Rev. 88 (4): 1547–1565. DOI: 10.1152/PHYSREV.00004.2008.
15. Rosenstock SJ, Jørgensen T (1995) Prevalence and incidence of peptic ulcer disease in a Danish County--a prospective cohort study. Gut 36 (6): 819–824. DOI: 10.1136/GUT.36.6.819.
16. Kurata JH, Nogawa AN, Abbey DE, Petersen F (1992) A prospective study of risk for peptic ulcer disease in Seventh-Day Adventists. Gastroenterology 102 (3): 902–909. DOI: 10.1016/0016-5085(92)90176-Y.
17. Lanas A, García-Rodríguez LA, Polo-Tomás M, Ponce M, Quintero E, Perez-Aisa MA, Gisbert JP, Bujanda L, Castro M, Muñoz M, Del-Pino MD, Garcia S, Calvet X (2011) The changing face of hospitalisation due to gastrointestinal bleeding and perforation. Aliment. Pharmacol. Ther. 33 (5): 585–591. DOI: 10.1111/J.1365-2036.2010.04563.X.
18. Malmi H, Kautiainen H, Virta LJ, Färkkilä N, Koskenpato J, Färkkilä MA (2014) Incidence and complications of peptic ulcer disease requiring hospitalisation have markedly decreased in Finland. Aliment. Pharmacol. Ther. 39 (5): 496–506. DOI: 10.1111/APT.12620.
19. Schwarz K (1910) Ueberpenetrierendemagen-und jejunalgeschwure. Beitr. Klin. Chir. 67 : 96–128.
20. Tripathi KD (2004) Essentials of medical pharmacology. Jaypee Brothers, Medical Publishers; 2004. 184–185 p. DOI: 10.5005/jp/books/12256.
21. Henry DA, Langman MJS (1981) Adverse effects of anti-ulcer drugs. Drugs 21 (6): 444–459. DOI: 10.2165/00003495-198121060-00004.
22. Nista EC, Candelli M, Cremonini F, Cazzato IA, Zocco MA, Franceschi F, Cammarota G, Gasbarrini G, Gasbarrini A (2004) Bacillus clausii therapy to reduce side-effects of anti-Helicobacter pylori treatment: randomized, double-blind, placebo controlled trial. Aliment. Pharmacol. \&Ther. 20 (10): 1181–1188. DOI: 10.1111/j.1365-2036.2004.02274.x.
23. Tanikawa C, Urabe Y, Matsuo K, Kubo M, Takahashi A, Ito H, Tajima K, Kamatani N, Nakamura Y, Matsuda K (2012) A genome-wide association study identifies two susceptibility loci for duodenal ulcer in the Japanese population. Nat. Genet. 44 (4): 430–434. DOI: 10.1038/NG.1109.
24. Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, Lam SK, Xiao SD, Tan HJ, Wu CY, Jung HC, Hoang BH, Kachintorn U, Goh KL, Chiba T, Rani AA (2009) Second asia-pacific consensus guidelines for Helicobacter pylori infection. J. Gastroenterol. Hepatol. 24 (10): 1587–1600. DOI: 10.1111/J.1440-1746.2009.05982.X.
25. Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH, Jones NL, Render C, Leontiadis GI, Moayyedi P, Marshall JK (2016) The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 151 (1): 51-69. e14. DOI: 10.1053/J.GASTRO.2016.04.006.
26. Malfertheiner P, Megraud F, O’Morain C, Gisbert JP, Kuipers EJ, Axon A et al. (2017) Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 66 (1): 6–30. DOI: 10.1136/GUTJNL-2016-312288.
27. Graham DY, laine l (2016) The Toronto Helicobacter pylori consensus in context. Gastroenterology 151 (1): 9. DOI: 10.1053/J.GASTRO.2016.05.009.
28. Leontiadis GI, Sreedharan A, Dorward S, Barton P, Delaney B, Howden CW, Orhewere M, Gisbert J, Sharma VK, Rostom A, Moayyedi P, Forman D (2007) Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding. Health Technol. Assess. 11 (51). DOI: 10.3310/HTA11510.
29. Wallace JL (1997) Nonsteroidal anti-inflammatory drugs and gastroenteropathy: The second hundred years. Gastroenterology 112 (3): 1000–1016. DOI: 10.1053/gast.1997.v112.pm9041264.
30. Perini R, Ma L, Wallace J (2003) Mucosal repair and COX-2 inhibition. Curr. Pharm. Des. 9 (27): 2207–2211. DOI: 10.2174/1381612033454027.
31. Hung LCT, Ching JYL, Sung JJY, To KF, Hui AJ, Wong VWS, Leong RWL, Chan HLY, Wu JCY, Leung WK, Lee YT, Chung SCS, Chan FKL (2005) Long-term outcome of helicobacter pylori-negative idiopathic bleeding ulcers: a prospective cohort study. Gastroenterology 128 (7): 1845–1850. DOI: 10.1053/J.GASTRO.2005.03.026.
32. Neil GA, Suchower LJ, Johnson E, Ronca PD, Skoglund ML (1998) Helicobacter pylori eradication as a surrogate marker for the reduction of duodenal ulcer recurrence. Aliment. Pharmacol. Ther. 12 (7): 619–633. DOI: 10.1046/J.1365-2036.1998.00351.X.
33. Fock KM, Talley N, Goh KL, Sugano K, Katelaris P, Holtmann G et al. (2016) Asia-Pacific consensus on the management of gastro-oesophageal reflux disease: an update focusing on refractory reflux disease and Barrett’s oesophagus. Gut 65 (9): 1402–1415. DOI: 10.1136/GUTJNL-2016-311715.
34. Tran-Duy A, Spaetgens B, Hoes AW, de Wit NJ, Stehouwer CDA (2016) Use of proton pump inhibitors and risks of fundic gland polyps and gastric cancer: systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 14 (12): 1706-1719.e5. DOI: 10.1016/J.CGH.2016.05.018.
35. Orlando LA, Lenard L, Orlando RC (2007) Chronic hypergastrinemia: causes and consequences. Dig. Dis. Sci. 52 (10): 2482–2489. DOI: 10.1007/S10620-006-9419-3.
36. Everhart JE, Ruhl CE (2009) Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology 136 (4): 1134–1144. DOI: 10.1053/J.GASTRO.2009.02.038.
37. DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ, Schulick RD (2007) Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann. Surg. 245 (5): 755–762. DOI: 10.1097/01.SLA.0000251366.62632.D3.
38. Fava G (2010) Molecular mechanisms of cholangiocarcinoma. World J. Gastrointest. Pathophysiol. 1 (1): 12. DOI: 10.4291/WJGP.V1.I1.12.
39. Razumilava N, Gores GJ (2013) Classification, diagnosis, and management of cholangiocarcinoma. Clin. Gastroenterol. Hepatol. 11 (1): 13-21.e1. DOI: 10.1016/j.cgh.2012.09.009.
40. Blechacz B, Komuta M, Roskams T, Gores GJ (2003) Clinical diagnosis and staging of cholangiocarcinoma. J. Hepatobiliary Pancreat Surg. 10 (4): 288–291. DOI: 10.1038/nrgastro.2011.131.
41. Nagorney DM, Donohue JH, Farnell MB, Schleck CD, Ilstrup DM (1993) Outcomes after curative resections of cholangiocarcinoma. Arch. Surg. 128 (8): 871–878. DOI: 10.1001/ARCHSURG.1993.01420200045008.
42. Blechacz B, Gores GJ (2008) Cholangiocarcinoma: Advances in pathogenesis, diagnosis, and treatment. Hepatology 48 (1): 308. DOI: 10.1002/hep.22310.
43. Cheung KS, Chan EW, Wong AYS, Chen L, Wong ICK, Leung WK (2018) Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population-based study. Gut 67 (1): 28–35. DOI: 10.1136/GUTJNL-2017-314605.
44. Aly A, Shulkes A, Baldwin GS (2004) Gastrins, cholecystokinins and gastrointestinal cancer. Biochim. Biophys. Acta. 1704 (1): 1–10. DOI: 10.1016/J.BBCAN.2004.01.004.
45. Welzel TM, Graubard BI, El-Serag HB, Shaib YH, Hsing AW, Davila JA, McGlynn KA (2007) Risk factors for intra- and extrahepatic cholangiocarcinoma in the United States: a population based case-control study. Clin. Gastroenterol. Hepatol. 5 (10): 1221. DOI: 10.1016/J.CGH.2007.05.020.
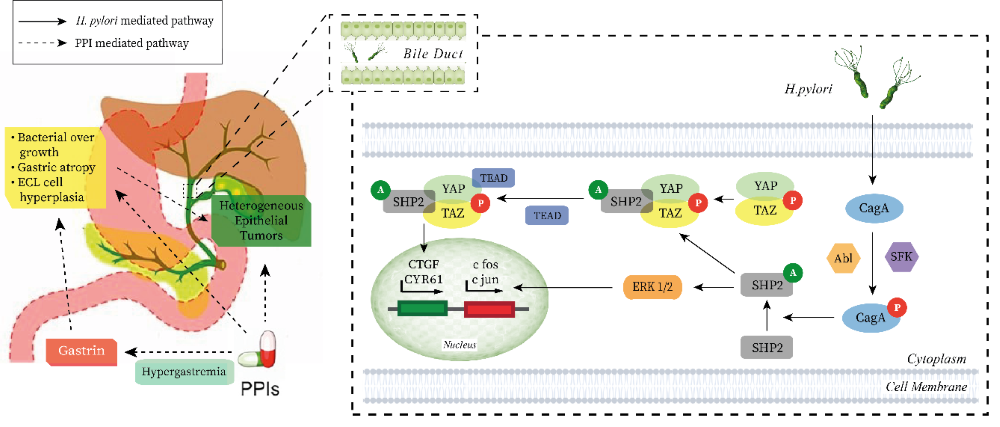
46. Kuroki T, Fukuda K, Yamanouchi K, Kitajima T, Matsuzaki S, Tajima Y, Furui J, Kanematsu T (2002) Helicobacter pylori accelerates the biliary epithelial cell proliferation activity in hepatolithiasis. Hepatogastroenterology 49 (45): 648–651. PMID: 12063961.
47. Narayanan M, Reddy KM, Marsicano E (2018) Peptic ulcer disease and Helicobacter pylori infection. Mo. Med. 115 (3): 219. PMID: 30228726.
48. Boonyanugomol W, Chomvarin C, Sripa B, Bhudhisawasdi V, Khuntikeo N, Hahnvajanawong C, Chamsuwan A (2012) Helicobacter pylori in Thai patients with cholangiocarcinoma and its association with biliary inflammation and proliferation. HPB (Oxford) 14 (3): 177. DOI: 10.1111/J.1477-2574.2011.00423.X.
49. Lee JH, Budanov A V., Karin M (2013) Sestrins orchestrate cellular metabolism to attenuate aging. Cell Metab. 18 (6): 792–801. DOI: 10.1016/j.cmet.2013.08.018.
50. Zhang J, Zhang F, Niu R (2015) Functions of Shp2 in cancer. J. Cell. Mol. Med. 19 (9): 2075–2083. DOI: 10.1111/JCMM.12618.
51. Poppe M, Feller SM, Römer G, Wessler S (2006) Phosphorylation of Helicobacter pylori CagA by c-Abl leads to cell motility. Oncogene 26 (24): 3462–3472. DOI: 10.1038/sj.onc.1210139.
52. Krisch LM, Posselt G, Hammerl P, Wessler S (2016) CagA phosphorylation in Helicobacter pylori-Infected B cells is mediated by the nonreceptor tyrosine kinases of the Src and Abl families. Infect. Immun. 84 (9): 2671. DOI: 10.1128/IAI.00349-16.
53. Adiseshaiah P, Li J, Vaz M, Kalvakolanu D V, Reddy SP (2008) ERK signaling regulates tumor promoter induced c-Jun recruitment at the Fra-1 promoter. Biochem. Biophys. Res. Commun. 371 (2): 304–308. DOI: 10.1016/j.bbrc.2008.04.063.
54. Pandey M (2007) Helicobacter species are associated with possible increase in risk of biliary lithiasis and benign biliary diseases. World J. Surg. Oncol. 5: 94. DOI: 10.1186/1477-7819-5-94.
55. Peek RM, Crabtree JE (2006) Helicobacter infection and gastric neoplasia. J. Pathol. 208 (2): 233–248. DOI: 10.1002/PATH.1868.
56. Myung SJ, Kim MH, Ki Nam Shim, Kim YS, Eun Ok Kim, Kim HJ, Park ET, Yoo KS, Lim BC, Dong Wan Seo, Sung Koo Lee, Young Il Min, Ji Yeon Kim (2000) Detection of Helicobacter pylori DNA in human biliary tree and its association with hepatolithiasis. Dig. Dis. Sci. 45 (7): 1405–1412. DOI: 10.1023/A:1005572507572.
57. Fortinsky KJ, Bardou M, Barkun AN (2015) Role of medical therapy for nonvariceal upper gastrointestinal bleeding. Gastrointest. Endosc. Clin. N. Am. 25 (3): 463–478. DOI: 10.1016/J.GIEC.2015.02.003.
58. Forgacs I, Loganayagam A (2008) Overprescribing proton pump inhibitors. Br. Med. J. 336 (7634): 2. DOI: 10.1136/BMJ.39406.449456.BE.
59. Yang YX, Metz DC (2010) Safety of proton pump inhibitor exposure. Gastroenterology 139 (4): 1115–1127. DOI: 10.1053/J.GASTRO.2010.08.023.
60. Poulsen AH, Christensen S, McLaughlin JK, Thomsen RW, Sørensen HT, Olsen JH, Friis S (2009) Proton pump inhibitors and risk of gastric cancer: a population-based cohort study. Br. J. Cancer. 100 (9): 1503–1507. DOI: 10.1038/SJ.BJC.6605024.
61. Chien LN, Huang YJ, Shao YHJ, Chang CJ, Chuang MT, Chiou HY, Yen Y (2016) Proton pump inhibitors and risk of periampullary cancers--A nested case-control study. Int. J. cancer 138 (6): 1401–1409. DOI: 10.1002/IJC.29896.
62. Xiong J, Wang Y, Xu W, Liu Z, Wang H, Zhang Z, Han Y, Yin C, Cao S, Yang Z, Su T, Wei J, Chen G, Jin L (2020) Proton pump inhibitors and odds of cholangiocarcinoma: A retrospective case-control study. Liver Int. 40 (11): 2848–2857. DOI: 10.1111/LIV.14663.
63. Kamal H, Sadr-Azodi O, Engstrand L, Brusselaers N (2021) Association between proton pump inhibitor use and biliary tract cancer risk: A Swedish population-based cohort study. Hepatology 74 (4): 2021–2031. DOI: 10.1002/HEP.31914.
64. Graham DY (2010) Treatment of peptic ulcers caused by Helicobacter pylori. N. Engl. J. Med. 328 (5): 349–350. DOI: 10.1056/NEJM199302043280512.
65. Redman J, Armstrong S, Ng KT (1983) Free-running activity rhythms in the rat: Entrainment by melatonin. Science 219 (4588): 1089–1091. DOI: 10.1126/science.6823571.
66. Srinivasan V, Maestroni GJM, Cardinali DP, Esquifino AI, PandiPerumal SR, Miller SC (2005) Melatonin, immune function and aging. Immun. Ageing. 2: 17. DOI: 10.1186/1742-4933-2-17.
67. Majumder R, Datta M, Pal PK, Bhattacharjee B, Chattopadhyay A, Bandyopadhyay D (2019) Protective mechanisms of melatonin on caprine spleen injury induced by cadmium (Cd): an in vitro study. Melatonin Res. 2 (3): 57–75. DOI: 10.32794/11250031.
68. Magierowski M, Jasnos K, Brzozowska I, Drozdowicz D, Sliwowski Z, Nawrot E, Szczyrk U, Kwiecień S (2013) Melatonin as a therapeutic factor in gastric ulcer healing under experimental diabetes. Przegl. Lek. 70 (11): 942–946. PMID: 24697035.
69. Celinski K, Konturek S, Slomka M, Cichoz-Lach H, Brzozowski T, Bielanski W, Konturek PC (2012) Effects of melatonin and tryptophan on healing of gastric and duodenal ulcers with Helicobacter pylori infection in humans. Gastroenterology 142 (5): S-491. PMID: 22204799.
70. Kato K, Murai I, Asai S, Takahashi Y, Nagata T, Komuro S, Mizuno S, Iwasaki A, Ishikawa K, Arakawa Y (2002) Circadian rhythm of melatonin and prostaglandin in modulation of stress-induced gastric mucosal lesions in rats. Aliment. Pharmacol. Ther. Suppl. 16 (2): 29–34. DOI: 10.1046/j.1365-2036.16.s2.11.x.
71. Majumder R, Datta M, Chattopadhyay A, Bandyopadhyay D (2021) Melatonin promotes gastric healing by modulating the components of matrix metalloproteinase signaling pathway: a novel scenario for gastric ulcer management. Melatonin Res. 4 (2): 213–231. DOI: 10.32794/mr11250092.
72. Brzozowska I, Strzalka M, Drozdowicz D, Konturek S, Brzozowski T (2014) Mechanisms of esophageal protection, gastroprotection and ulcer healing by melatonin. implications for the therapeutic use of melatonin in gastroesophageal reflux disease (GERD) and peptic ulcer disease. Curr. Pharm. Des. 20 (30): 4807–4815. DOI: 10.2174/1381612819666131119110258.
73. Brzozowska I, Ptak-Belowska A, Pawlik M, Pajdo R, Drozdowicz D, Konturek S, Pawlik W, Brzozowski T (2009) Mucosal strengthening activity of central and peripheral melatonin in the mechanism of gastric defense. J. Physiol. Pharmacol. 60 (Suppl 7): 47-56. PMID: 20388945.
74. Han Y, DeMorrow S, Invernizzi P, Jing Q, Glaser S, Renzi A, Meng F, Venter J, Bernuzzi F, White M, Francis H, Lleo A, Marzioni M, Onori P, Alvaro D, Torzilli G, Gaudio E, Alpini G (2011) Melatonin exerts by an autocrine loop antiproliferative effects in cholangiocarcinoma; its synthesis is reduced favoring cholangiocarcinoma growth. Am. J. Physiol. - Gastrointest. Liver Physiol. 301 (4): G623. DOI: 10.1152/AJPGI.00118.2011.
75. Laothong U, Hiraku Y, Oikawa S, Intuyod K, Murata M, Pinlaor S (2015) Melatonin induces apoptosis in cholangiocarcinoma cell lines by activating the reactive oxygen species-mediated mitochondrial pathway. Oncol. Rep. 33 (3): 1443–1449. DOI: 10.3892/OR.2015.3738.
76. Qureshi W, Graham D (2000) Antibiotic-resistant H. pylori infection and its treatment. Curr. Pharm. Des. 6 (15): 1537–1544. DOI: 10.2174/1381612003399077.
77. Chojnacki C, Mędrek-Socha M, Konrad P, Chojnacki J, Błońska A (2020) The value of melatonin supplementation in postmenopausal women with Helicobacter pylori-associated dyspepsia. BMC Womens Health 20 (1): 1–6. httpsDOI: 10.1186/s12905-020-01117-z.
78. Tekbas OF, Ogur R, Korkmaz A, Kilic A, Reiter RJ (2008) Melatonin as an antibiotic: new insights into the actions of this ubiquitous molecule. J. Pineal Res. 44 (2): 222–226. DOI: 10.1111/J.1600-079X.2007.00516.X.
79. Rothfield L, Horecker BL (1964) The role of cell-wall lipid in the biosynthesis of bacterial lipopolysaccharide. Proc. Natl. Acad. Sci. U. S. A. 52 (4): 939. DOI: 10.1073/PNAS.52.4.939.
80. Hebeler BH, Chatterjee AN, Young FE (1973) Regulation of the bacterial cell wall: effect of antibiotics on lipid biosynthesis. Antimicrob. Agents Chemother. 4 (3): 346–353. DOI: 10.1128/AAC.4.3.346.
81. Konar V, Yilmaz Ö, Öztürk AI, Kirbaǧ S, Arslan M (2000) Antimicrobial and biological effects of bomphos and phomphos on bacterial and yeast cells. Bioorg. Chem. 28 (4): 214–225. DOI: 10.1006/BIOO.2000.1173.
82. Limson J, Nyokong T, Daya S (1998) The interaction of melatonin and its precursors with aluminium, cadmium, copper, iron, lead, and zinc: an adsorptive voltammetric study. J. Pineal Res. 24 (1): 15–21. DOI: 10.1111/J.1600-079X.1998.TB00361.X.
83. Tan DX, Manchester LC, Reiter RJ, Qi W, Hanes MA, Farley NJ (1999) High physiological levels of melatonin in the bile of mammals. Life Sci. 65 (23): 2523–2529. DOI: 10.1016/s0024-3205(99)00519-6.
84. Chojnacki C, Popławski T, Blasiak J, Chojnacki J, Reiter RJ, Klupinska G (2013) Expression of melatonin synthesizing enzymes in Helicobacter pylori infected gastric mucosa. Biomed Res. Int. 2013: 845032. DOI: 10.1155/2013/845032.
85. Lai L, Yuan L, Cheng Q, Dong C, Mao L, Hill SM (2008) Alteration of the MT1 melatonin receptor gene and its expression in primary human breast tumors and breast cancer cell lines. Breast Cancer Res. Treat. 118 (2): 293–305. DOI: 10.1007/S10549-008-0220-1.
86. Mortezaee K, Najafi M, Farhood B, Ahmadi A, Potes Y, Shabeeb D, Musa AE (2019) Modulation of apoptosis by melatonin for improving cancer treatment efficiency: An updated review. Life Sci. 228: 228–241.DOI: 10.1016/j.lfs.2019.05.009.
87. Torres JDF de O, Pereira R de S (2010) Which is the best choice for gastroesophageal disorders: Melatonin or proton pump inhibitors? World J. Gastrointest. Pharmacol. Ther. 1 (5): 102. DOI: 10.4292/WJGPT.V1.I5.102.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


