A combination of melatonin and moderate-intensity aerobic exercise improves pancreatic beta-cell function and glycemic homeostasis in type 2 diabetic model of animals
Melatonin and aerobic exercise improves pancreatic beta-cell function and glycemic homeostasis
Abstract
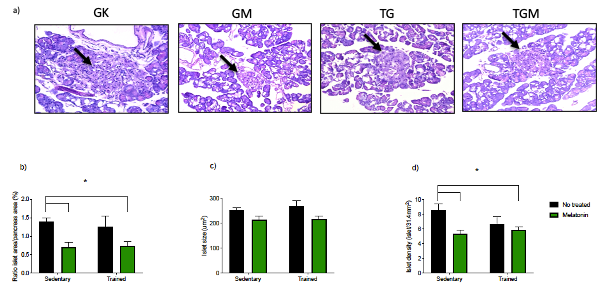
Nocturnal melatonin secretion is important for preservation of ß-cell mass and function. Knowing that type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder characterized by hyperglycemia caused by the elevated resistance of peripheral tissues to insulin, reduction in pineal melatonin and disturbances of insulin secretion by pancreatic ß-cells. In this context, exercise is considered one of the most valuable non-pharmacological approaches for treatment of T2DM. Considering the beneficial role of melatonin on glycemic metabolism in physical exercise, we investigated the effects of moderate-intensity aerobic exercise plus melatonin on glycemic homeostasis, the morphology and architecture of pancreas in spontaneous T2DM animals [Goto-Kakizaki (GK) rats]. The results confirmed that melatonin alone reduced the mass of epididymal white adipose tissue (WAT); however, only the combination of melatonin and physical exercise significantly reduced caloric intake, body weight, WAT and improved glucose tolerance and insulin sensitivity in T2DM rats. This combination also reduced apoptosis of cells in pancreatic islets. We observed either melatonin or the combination was able to reduce insulinemia. However, only the combination improved the morphology of the pancreatic islets. Thus, we conclude that in GK rats, melatonin plays a crucial role in the functionality of the pancreas to improve insulin sensitivity of peripheral tissues and, consequently, to maintain the glucose homeostasis. In addition, the combination is more efficiency to improve glucose tolerance and integrity of pancreatic islets in GK rats than melatonin alone.
References
2. Peliciari-Garcia RA et al. (2010) Insulin temporal sensitivity and its signaling pathway in the rat pineal gland. Life Sci. 87: 169-174.
3. Picinato MC et al. (2002) Melatonin inhibits insulin secretion and decreases PKA levels without interfering with glucose metabolism in rat pancreatic islets. J Pineal Res. 33: 156-160.
4. Costes S, Boss M, Thomas AP, Matveyenko AV (2015) Activation of melatonin signaling promotes beta-cell survival and function. Mol. endocrinol. 29: 682-692.
5. Cipolla-Neto J, Amaral FGD. (2018) Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 39: 990-1028.
6. Picinato MC et al. (2008) Activation of insulin and IGF-1 signaling pathways by melatonin through MT1 receptor in isolated rat pancreatic islets. J Pineal Res. 44, 88-94.
7. American Diabetes Association (2017) Classification and Diagnosis of Diabetes. Diabetes care 40 (Supplement 1): S11-S24.
8. Mellado C, Rodríguez V, Diego JG, Alvarez E, Blázquez E (1989) Effect of pinealectomy and of diabetes on liver insulin and glucagon receptor concentrations in the rat. J Pineal Res. 6: 295-306.
9. Mantele S et al. (2012) Daily rhythms of plasma melatonin, but not plasma leptin or leptin mRNA, vary between lean, obese and type 2 diabetic men. PloS one 7: e37123.
10. Peschke E et al. (2006) Diabetic Goto Kakizaki rats as well as type 2 diabetic patients show a decreased diurnal serum melatonin level and an increased pancreatic melatonin-receptor status. J. Pineal Res. 40: 135-143.
11. Movassat J et al. (2007) Type 2 diabetes - a matter of failing beta-cell neogenesis? Clues from the GK rat model. Diabetes Obes Metab. 9 (Suppl 2): 187-195.
12. Ehses JA et al. (2007) Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 56: 2356-2370.
13. Portha B et al. (2009) The GK rat beta-cell: a prototype for the diseased human beta-cell in type 2 diabetes? Mol. Cell Endocrinol. 297: 73-85.
14. Giroix MH et al. (2011) Hypercholesterolaemia, signs of islet microangiopathy and altered angiogenesis precede onset of type 2 diabetes in the Goto-Kakizaki (GK) rat. Diabetologia 54: 2451-2462.
15. Portha B et al. (2007) Defective functional β-cell mass and Type 2 diabetes in the Goto-Kakizaki rat model. Expert Rev. Endocrinol. Metab. 2: 785-795.
16. Portha B (2005) Programmed disorders of beta-cell development and function as one cause for type 2 diabetes? The GK rat paradigm. Diabetes Metab. Res. Rev. 21: 495-504.
17. Kuwabara WMT et al. (2017) Comparison of Goto-Kakizaki rats and high fat diet-induced obese rats: Are they reliable models to study type 2 diabetes mellitus? PLoS One 12: e0189622.
18. Picarel-Blanchot F, Berthelier C, Bailbé D, Portha B (1996) Impaired insulin secretion and excessive hepatic glucose production are both early events in the diabetic GK rat. Am. J. Physiol. 271: E755-762.
19. Movassat J, Saulnier C, Serradas P, Portha B (1997) Impaired development of pancreatic beta-cell mass is a primary event during the progression to diabetes in the GK rat. Diabetologia 40: 916-925.
20. Portha B et al. (2001) beta-cell function and viability in the spontaneously diabetic GK rat: information from the GK/Par colony. Diabetes 50 (Suppl 1): S89-93.
21. Portha B, Giroix MH, Tourrel-Cuzin C, Le-Stunff H, Movassat J (2012) The GK rat: a prototype for the study of non-overweight type 2 diabetes. Methods Mol. Biol. 933: 125-159.
22. Raza H, John A , Shafarin J , Howarth FC (2016) Howarth, Exercise-induced alterations in pancreatic oxidative stress and mitochondrial function in type 2 diabetic Goto-Kakizaki rats. Physiol. Rep. 4 (8): e12751.
23. Knowler WC et al. (2002) Reduction in the incidence of type 2 diabetes with life style intervention or metformin. N. Engl. J. Med. 346: 393–403.
24. Rawal S et al. (2013) Effects of exercise on pancreatic islets in zucker diabetic fatty rats. J Diabetes Metab. S10: 007.
25. Paula FMM et al. (2015) Exercise increases pancreatic beta-cell viability in a model of type 1 diabetes through IL-6 signaling. FASEB J. 29: 1805-1816.
26. Borges-Silva CN et al. (2005) Pinealectomy impairs adipose tissue adaptability to exercise in rats. J Pineal Res. 38: 278-283.
27. Mendes C et al. (2013) Adaptations of the aging animal to exercise: role of daily supplementation with melatonin. J. Pineal Res. 55: 229-239.
28. Goto Y, Kakizaki M, Masaki N (1976) Production of spontaneous diabetic rats by repetition of selective breeding. Tohoku J. Exp. Med. 119: 85-90.
29. Buxton OM et al. (1997) Roles of intensity and duration of nocturnal exercise in causing phase delays of human circadian rhythms. Am. J. Physiol. 273: E536-542.
30. Brooks GA, White TP. (1978) Determination of metabolic and heart rate responses of rats to treadmill exercise. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 45: 1009-1015.
31. Bonora E, Manicardi V, Zavaroni I, Coscelli C, Butturini U (1987) Relationships between insulin secretion, insulin metabolism and insulin resistance in mild glucose intolerance. Diabete Metab. 13: 116-121.
32. Gomes PRL et al. (2021) Melatonin regulates maternal pancreatic remodeling and B-cell function during pregnancy and lactation. J. Pineal Res. 71 (1): e12717. doi: 10.1111/jpi.12717.
33. Weibel ER (1063) Principles and methods for the morphometric study of the lung and other organs. Lab. Invest. 12: 131-155 .
34. Choi EY et al. (2020) Association between clinical biomarkers and optical coherence tomography angiography parameters in type 2 diabetes mellitus. Invest. Ophthalmol. Vis. Sci. 61: 4.
35. Pan XR et al. (1997) Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 20: 537-544.
36. Tuomilehto J et al. (2001) Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 344: 1343-1350.
37. Knowler WC et al. (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 346: 393-403.
38. Knowler WC et al. (2009) 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 374: 1677-1686.
39. Cipolla-Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ (2014) Melatonin, energy metabolism, and obesity: a review. J. Pineal Res. 56: 371-381.
40. Lumb A (2014) Diabetes and exercise. Clin. Med. 14: 673-676.
41. Frese T et al. (2009) Pineal melatonin synthesis is decreased in type 2 diabetic Goto-Kakizaki rats. Life Sci. 85: 526-533.
42. Buonfiglio D et al. (2018) Melatonin absence leads to long-term leptin resistance and overweight in rats. Front Endocrinol. 9: 122.
43. Tan DX, Manchester LC, Fuentes-Broto L, Paredes SD, Reiter RJ. (2011) Significance and application of melatonin in the regulation of brown adipose tissue metabolism: relation to human obesity. Obes. Rev. 12: 167-188.
44. Prunet-Marcassus B et al. (2003) Melatonin reduces body weight gain in Sprague Dawley rats with diet-induced obesity. Endocrinology 144: 5347-5352.
45. Sartori C et al. (2009) Melatonin improves glucose homeostasis and endothelial vascular function in high-fat diet-fed insulin-resistant mice. Endocrinology 150: 5311-5317.
46. Evans WJ, Cyr-Campbell D (1997) Nutrition, exercise, and healthy aging. J. Am. Diet Assoc. 97: 632-638.
47. Borges-Silva C et al. (2007) Pinealectomy reduces hepatic and muscular glycogen content and attenuates aerobic power adaptability in trained rats. J. Pineal Res. 43: 96-103.
48. Scheer FA, Hilton MF, Mantzoros CS, Shea SA (2009) Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. 106: 4453-4458.
49. Picinato MC, Haber EP, Carpinelli AR, Cipolla-Neto J (2002) Daily rhythm of glucose-induced insulin secretion by isolated islets from intact and pinealectomized rat. J. Pineal Res. 33: 172-177.
50. Topp BG, Atkinson LL, Finegood DT (2007) Dynamics of insulin sensitivity, -cell function, and -cell mass during the development of diabetes in fa/fa rats. Am. J. Physiol. Endocrinol. Metab. 293: E1730-1735.
51. Butler AE et al. (2003) Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52: 102-110.
52. Porte D (1996) Normal physiology and phenotypic characterization of beta-cell function in subjects at risk for non-insulin-dependent diabetes mellitus. Diabete Med. 13: S25-32.
53. Vinik A (2007) Advancing therapy in type 2 diabetes mellitus with early, comprehensive progression from oral agents to insulin therapy. Clin. Ther. 29: 1236-1253.
54. Park S, Hong SM, Lee JE, Sung SR (2007) Exercise improves glucose homeostasis that has been impaired by a high-fat diet by potentiating pancreatic beta-cell function and mass through IRS2 in diabetic rats. J. Appl. Physiol. 103: 1764-1771.
55. Duclos M, Corcuff JB, Pehourcq F, Tabarin A (2001) Decreased pituitary sensitivity to glucocorticoids in endurance-trained men. Eur. J. Endocrinol. 144: 363-368.
56. Campbell JE, Rakhshani N, Fediuc S, Bruni S, Riddell MC (2009) Voluntary wheel running initially increases adrenal sensitivity to adrenocorticotrophic hormone, which is attenuated with long-term training. J. Appl. Physiol. 106: 66-72.
57. Droste SK, Chandramohan Y, Hill LE, Linthorst ACE, Reul JMHM (2007) Voluntary exercise impacts on the rat hypothalamic-pituitary-adrenocortical axis mainly at the adrenal level. Neuroendocrinology 86: 26-37.
58. Coutinho AE, Campbell JE, Fediuc S, Riddell MC (2006) Effect of voluntary exercise on peripheral tissue glucocorticoid receptor content and the expression and activity of 11beta-HSD1 in the Syrian hamster. J. Appl. Physiol. 100: 1483-1488.
59. Campbell JE et al. (2010) Regular exercise prevents the development of hyperglucocorticoidemia via adaptations in the brain and adrenal glands in male Zucker diabetic fatty rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299: R168-176.
60. Heath GW et al. (1983) Effects of exercise and lack of exercise on glucose tolerance and insulin sensitivity. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 55: 512-517.
61. Nilsen V, Bakke PS, Gallefoss F ( 2011) Effects of lifestyle intervention in persons at risk for type 2 diabetes mellitus - results from a randomised, controlled trial. BMC Public Health 11: 893.
62. Burr JF, Rowan CP, Jamnik VK, Riddell MC (2010) The role of physical activity in type 2 diabetes prevention: physiological and practical perspectives. Phys. Sportsmed. 38: 72-82.
63. Richter EA, Mikines KJ, Galbo H, Kiens B (1989) Effect of exercise on insulin action in human skeletal muscle. J. Appl. Physiol. 66: 876-885.
64. Beaudry JL, Riddell MC (2012) Effects of glucocorticoids and exercise on pancreatic β-cell function and diabetes development. Diabetes Metab. Res. Rev. 28: 560-573.
65. Costes S, Boss M, Thomas AP, Matveyenko AV (2015) Activation of melatonin signaling promotes β-cell survival and function. Mol. Endocrinol. 29: 682-692.
66. Peschke E et al. (2000) Evidence for a melatonin receptor within pancreatic islets of neonate rats: functional, autoradiographic, and molecular investigations. J. Pineal Res. 28: 156-164.
67. Mühlbauer E, Peschke E (2007) Evidence for the expression of both the MT1- and in addition, the MT2-melatonin receptor, in the rat pancreas, islet and beta-cell. J. Pineal Res. 42: 105-106.
68. Stumpf I, Bazwinsky I, Peschke E (2009) Modulation of the cGMP signaling pathway by melatonin in pancreatic beta-cells. J. Pineal Res. 46: 140-147.
69. Stumpf I, Mühlbauer E, Peschke E (2008) Involvement of the cGMP pathway in mediating the insulin-inhibitory effect of melatonin in pancreatic beta-cells. J. Pineal Res. 45: 318-327.
70. Mühlbauer E, Albrecht E, Bazwinsky-Wutschke I, Peschke E (2012) Melatonin influences insulin secretion primarily via MT(1) receptors in rat insulinoma cells (INS-1) and mouse pancreatic islets. J. Pineal Res. 52: 446-459.
71. Peschke E et al. (2002) Receptor (MT(1)) mediated influence of melatonin on cAMP concentration and insulin secretion of rat insulinoma cells INS-1. J. Pineal Res. 33: 63-71.
72. Peschke E, D Peschke (1998) Evidence for a circadian rhythm of insulin release from perifused rat pancreatic islets. Diabetologia 41: 1085-1092.
73. Peschke E, D Peschke, T Hammer, V Csernus (1997) Csernus, Influence of melatonin and serotonin on glucose-stimulated insulin release from perifused rat pancreatic islets in vitro. J. Pineal Res. 23: 156-163.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


