Antinociceptive effect of melatonin in the animal model of Parkinson’s Disease
Antinociceptive effect of melatonin in Parkinson’s disease
Abstract
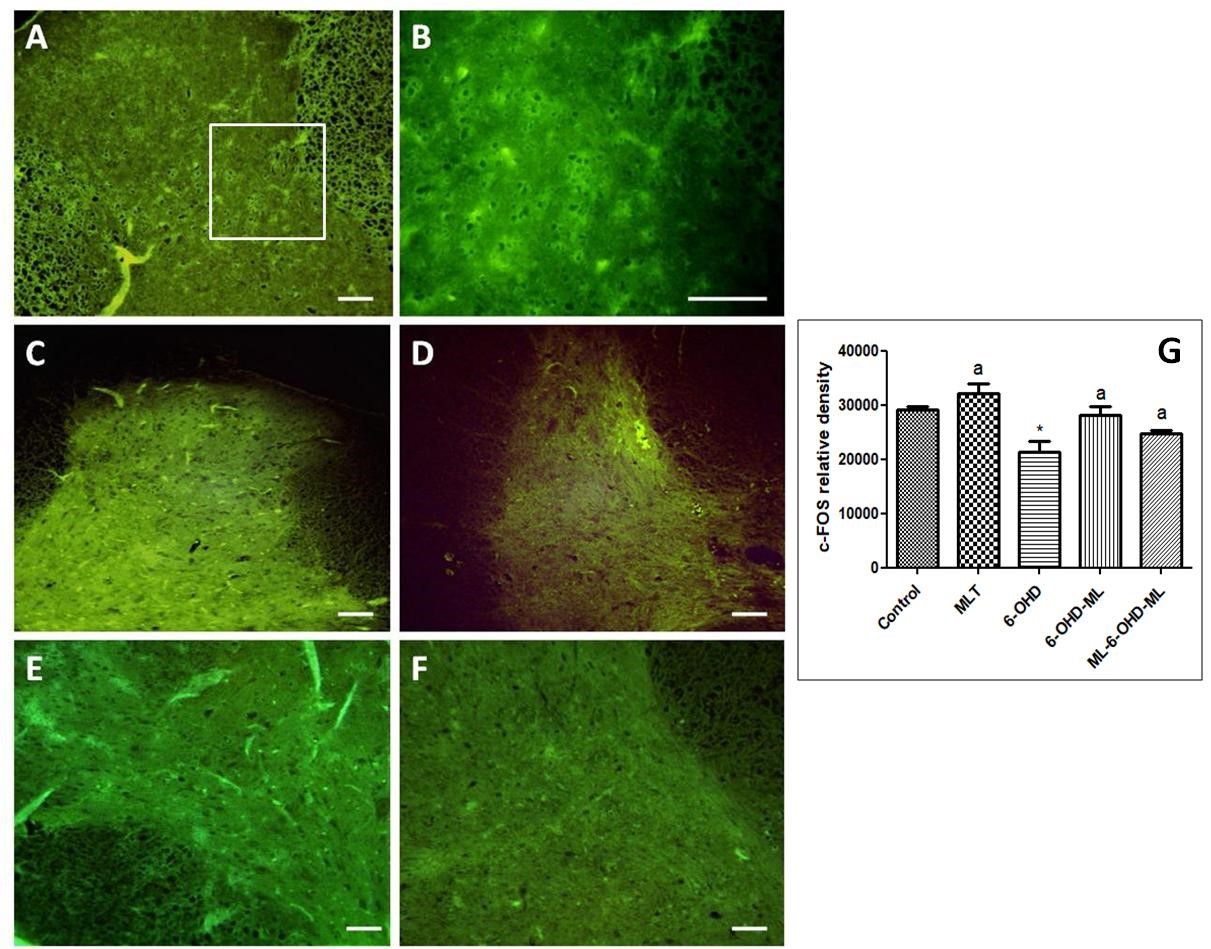
Several animal experimental and clinical studies have shown the effectiveness of melatonin in the treatment of some symptoms of Parkinson's disease (PD). However, the antinociceptive effect of melatonin against pain associated to PD has not been fully investigated. Thus, the present study investigated the possible antiallodynic and antinociceptive effects of acute and chronic melatonin treatments in Parkinsonian model of rats. This model was created by unilateral injection of 6-hydroxydopamine (6-OHDA) into the left medial forebrain bundle (MFB). The electronic von Frey test was used to analyze the antiallodynic effect of melatonin on this PD animal model. In addition, c-Fos immunostaining was also used as a marker of nociception to evaluate the neuronal activity related to the nociception processing. The results showed that unilateral injection of 6-OHDA induced a significant decrease in paw withdrawal threshold in both ipsilateral and contralateral paws, which indicate mechanical allodynia induction. This allodynia was transitorily reversed by apomorphine as a dopamine agonist. Melatonin treatment significantly increased threshold of allodynia. Melatonin administration of both acutely or chronically significantly downregulated the c-Fos expression of neurons in 6-OHDA treated animals. In conclusion, 6-OHDA treatment can induces a bilateral mechanical hypernociception in rats while melatonin treatment produces profound antinociceptive effect. This finding paves the way to use melatonin as an antinociceptive agent for PD clinically.
References
2. Olanow CW, Tatton WG (1999) Etiology and pathogenesis of Parkinson’s disease. Annu. Rev. Neurosci. 22: 123–144. https://doi.org/10.1146/annurev.neuro.22.1.123.
3. Zengin-Toktas Y, Ferrier J, Durif F, Llorca PM, Authier N (2013) Bilateral lesions of the nigrostriatal pathways are associated with chronic mechanical pain hypersensitivity in rats. Neurosci. Res. 76: 261–264. https://doi.org/10.1016/j.neures.2013.05.003.
4. Truini A, Frontoni M, Cruccu G (2013). Parkinson's disease-related pain: a review of recent findings. J. Neurol. 260: 330–334. DOI 10.1007/s00415-012-6754-5.
5. Crockett, RS, Bornchein, RL, Smith RP (1977). Diurnal variations in response to thermal stimulation: mouse-hot plate test. Physiol. Behav. 18 (2): 193-196. https://doi: 10.1016/0031-9384(77)90120-2.
6. Junker U, Wirz S (2010) Review article: Chronobiology: Influence of circadian rhythms on the therapy of severe pain. J. Oncol. Pharm. Pract. 16: 81–87. https://doi.org/10.1177/1078155209337665.
7. Laikin MI, Miller CH, Stott ML, Winters WD (1981) Involvement of the pineal gland and melatonin in murine analgesia. Life Sci. 29: 2543–2551. https://doi.org/10.1016/0024-3205(81)90710-4.
8. Pickard GE (1987) Circadian rhythm of nociception in the golden hamster. Brain Res. 425: 395–400. https://doi.org/10.1016/0006-8993(87)90529-4.
9. Srinivasan V, Pandi-Perumal SR, Spence DW, Moscovitch A, Trakht I, Brown GM, Cardinali DP (2010) Potential use of melatonergic drugs in analgesia: Mechanisms of action. Brain Res. Bull. 81: 362–371. https://doi.org/10.1016/j.brainresbull.2009.12.001.
10. Citera G, Arias MA, Maldonado-Cocco JA et al (2000). The effect of melatonin in patients with fibromyalgia: a pilot study. Clin. Rheumatol. 19: 9–13. https://doi.org/10.1007/s100670050003.
11. Srinivasan V, Spence DW, Pandi-Perumal SR, Brown GM, Cardinali DP (2011) Melatonin in mitochondrial dysfunction and related disorders. Int. J. Alzheimers Dis. 2011: 326320. https://doi.org/10.4061/2011/326320.
12. Breen DP, Nombela C, Vuono R, Jones PS, Fisher K, Burn DJ, Brooks DJ, Reddy AB, Rowe JB, Barker RA (2016) Hypothalamic volume loss is associated with reduced melatonin output in Parkinson’s disease. Mov. Disord. 31 (7): 1062–1066. https://doi.org/10.1002/mds.26592.
13. Leon J, Acuna-Castroviejo D, Sainz RM, Mayo JC, Tan DX, Reiter RJ (2004) Melatonin and mitochondrial function. Life Sci. 75: 765–790. https://doi.org/10.1016/j.lfs.2004.03.003.
14. Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, Martín V, Reiter RJ (2004). Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal Res. 36: 1–9. https://doi.org/10.1046/j.1600-079X.2003.00092.x.
15. Galano A, Tan DX, Reiter RJ (2011) Melatonin as a natural ally against oxidative stress: a physicochemical examination. J. Pineal Res. 51: 1–16. https://doi.org/10.1111/j.1600-079X.2011.00916.x.
16. Singhal NK, Srivastava G, Patel DK, Jain SK, Singh MP (2011) Melatonin or silymarin reduces maneb- and paraquat-induced Parkinson’s disease phenotype in the mouse. J. Pineal Res. 50: 97–109. https://doi.org/10.1111/j.1600-079X.2010.00819.x.
17. Kunz D, Mahlberg R (2010). A two-part, double-blind, placebo-controlled trial of exogenous melatonin in REM sleep behaviour disorder. J. Sleep Res.19 (4): 591–596. https://doi.org/10.1111/j.1365-2869.2010.00848.x
18. Dowling GA, Mastick J, Colling E, Carter JH, Singer CM, Aminoff MJ (2005). Melatonin for sleep disturbances in Parkinson’s disease. Sleep Med. 6 (5): 459–466. https://doi.org/10.1016/j.sleep.2005.04.004.
19. Kelly PH, Iversen SD (1976) Selective 6OHDA-induced destruction of mesolimbic dopamine neurons: abolition of psycho-stimulant-induced locomotor activity in rats. Eur. J. Pharmacol. 40: 45–56. https://doi.org/10.1016/0014-2999(76)90352-6.
20. Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates. Compact 6th Edition, Academic Press. New York 400.
21. Czarnecka A, Tomasz L, Domin H, Konieczny J, Smiałowska M, Lorenc-Kocia E (2013) Alterations in the expression of nNOS in the substantia nigra and subthalamic nucleus of 6-OHDA-lesioned rats: The effects of chronic treatment with L-DOPA and the nitric oxide donor, molsidomine. Brain Res. 1541: 92–105. https://doi.org/10.1016/j.brainres.2013.10.011.
22. Wu FS, Yang YC, Tsai JJ (2000) Non competitive inhibition of the glycine receptor-mediated current by melatonin in cultured neurons. Brain Res. 881: 208–211. https://doi.org/10.1016/S0006-8993(00)02804-3.
23. Mickle A, Sood M, Zhang Z, Shahmohammadi G, Sengupta JN, Miranda A (2010) Antinociceptive effects of melatonin in a rat model of post inflammatory visceral hyperalgesia: a centrally mediated process. Pain 149: 555–564. doi:10.1016/j.pain.2010.03.030.
24. Laste G, Macedo IC, Rozisky JR, da Silva FR, Iraci WC Torres LS (2012) Melatonin administration reduces inflammatory pain in rats. J. Pain Res. 5: 359–362. https://doi.org/10.2147/JPR.S34019.
25. Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST (2000) CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, Parkinsonism and spinal cord injury. Neuropharmacology 39: 777–787. https://doi.org/10.1016/S0028-3908(00)00005-8.
26. Thibault K, Elisabeth B, Sophie D, Claude FZ, Bernard R et al. (2008) Antinociceptive and anti-allodynic effects of oral PL37, a complete inhibitor of enkephalin-catabolizing enzymes, in a rat model of peripheral neuropathic pain induced by vincristine. Eur. J. Pharmacol. 600: 71–77. https://doi.org/10.1016/j.ejphar.2008.10.004.
27. Park J, Lim CS, Seo H, Park CA, Zhuo M et al. (2015) Pain perception in acute model mice of Parkinson's disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Mol. Pain 17: 11–28. https://doi.org/10.1186/s12990-015-0026-1.
28. Richner M, Jager SB, Siupka P, Vaegter CB (2017) Hydraulic extrusion of the spinal cord and isolation of dorsal root ganglia in rodents. J. Vis. Exp. 119: e55226. https://doi.org/10.3791/55226.
29. Shi YB, Gelman BG, Lisinicchia J, Tang S.J. (2012) Chronic-pain-associated astrocytic reaction in the spinal cord dorsal horn of human immunodeficiency virus-infected patients. J. Neurosci. 32 (32): 10833–10840. https://doi.org/10.1523/JNEUROSCI.5628-11.2012.
30. Carey RJ (1986) Acute ipsilateral hyperalgesia and chronic contralateral hypoalgesia after unilateral 6-hydroxydopamine lesions of the substantia nigra. Exp. Neurol. 91: 277–284.
31. Saade NF, Atweh SF, Bahuth NB, Jabbur SJ, (1997) Augmentation of nociceptive lesions of either dopaminergic terminals or midbrain dopaminergic neurons. Brain Res. 751: 1–12.
32. Chudler EH, Lu Y (2008) Nociceptive behavioral responses to chemical, thermal and mechanical stimulation after unilateral intrastriatal administration of 6-hydroxydopamine. Brain Res. 1213: 41–47. https://doi.org/10.1016/j.brainres.2008.03.053.
33. Carta M, Carlsson T, Kirik D, Bjorklund A (2007) Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain 130: 1819–1833. https://doi.org/10.1093/brain/awm082.
34. Domenici RA, Campos ACP, Maciel ST, Berzuino MB, Hernandes MS, Erich T, Fonoff ET, Pagano RL (2019) Parkinson's disease and pain: Modulation of nociceptive circuitry in a rat model of nigrostriatal lesion. Exp. Neurol. 315: 72–81. https://doi.org/10.1016/j.expneurol.2019.02.007.
35. Saidi N, Nsibi A, Mani S, Saoud H, Messaoudi I (2019) Unilateral 6-hydroxydopamine-lesioned rat as relevant model to study the pain related to parkinson’s disease. Neurol. Neurobiol. 1: 2613-7828. http://dx.doi.org/10.31487/j.NNB.2019.04.03.
36. Magnusson JE, Fisher K (2000) The involvement of dopamine in nociception: the role of D1 and D2 receptors in the dorsolateral striatum. Brain Res. 855: 260–266. https://doi.org/10.1016/S0006-8993(99)02396-3.
37. Rascol O, Lozano A, Stern M, Poewe W (2011) Milestones in Parkinson’s disease therapeutics. Mov. Disord. 26: 1072–1082. https://doi.org/10.1002/mds.23714.
38. Pérez-Lloret S, Cardinali DP (2021) Melatonin as a chronobiotic and cytoprotective agent in Parkinson’s Disease. Front. Paharmacol. 12: 650597. doi: 10.3389/fphar.2021.650597.
39. Lahiri DK (1999) Melatonin affects the metabolism of the

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


