Melatonin attenuates microglial activation and improves neurological functions in rat model of collagenase-induced intracerebral hemorrhage
Melatonin in intracerebral hemorrhage
Abstract
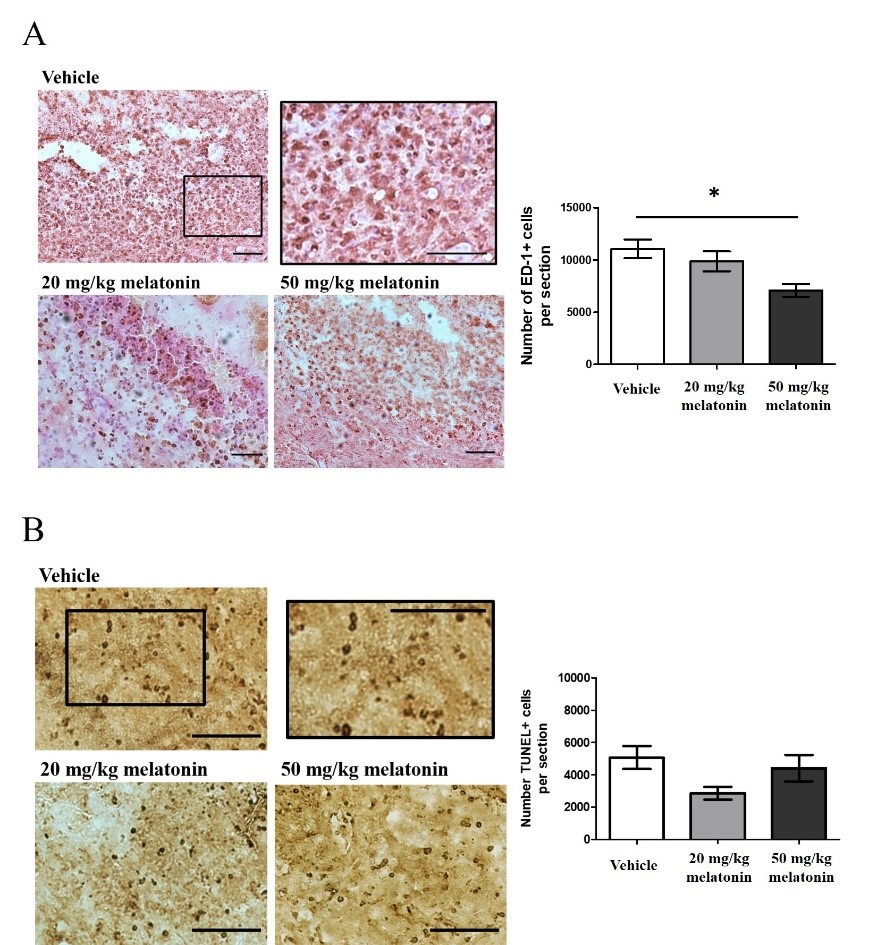
Intracerebral hemorrhage (ICH) is a severe form of stroke with a high mortality rate. It is also an important cause of permanent disability. Apart from hematoma growth and edema development, inflammatory responses and oxidative stress are responsible for poor outcomes after ICH. Due to its antioxidant, anti-inflammatory and anti-apoptotic properties, melatonin is a neuroprotective molecule against different neurological diseases. The protective roles of melatonin on ICH, particularly in the collagenase-induced ICH model, have not been well studied. The present study aims to explore neuroprotective effects of melatonin against ICH. At 24 hours after ICH induction, rats exhibited neurological deficits with mild loss in body weight (BW). Hematoma was found in the brain parenchyma with ED-1+ activated microglia and TUNEL+ apoptotic cells in the perihematomal region. As an in vitro model of ICH, SH-SY5Y cells were treated with red blood cell lysate. This treatment significantly reduced cell viability; however, melatonin (10-5 M) restored the cell viability. At 72 hours after ICH, rats treated with melatonin (50 mg/kg) at 2, 24 and 48 hours had reduced perihematomal microglial activation. However, there was no effect on hematoma size or perihematomal apoptosis. We further treated rats with 50 mg/kg melatonin starting at 2 hours and repeating at 24-hour intervals for two or seven more days. Both melatonin treatments improved post-ICH neurological functions, and the effect was most pronounced at 4 days after ICH. Since studies regarding the protective roles of melatonin on ICH remain very limited, our study advances our understanding of the potential use of melatonin as a treatment for ICH.
References
2. Wu H-J, Wu C, Niu H-J, Wang K, Mo L-J, Shao A-W, Dixon BJ, Zhang J-M, Yang S-X, Wang Y-R (2017) Neuroprotective mechanisms of melatonin in hemorrhagic stroke. Cell Mol. Neurobiol. 37: 1173–1185.
3. Kojic B, Burina A, Hodzic R, Pasic Z, Sinanovic O (2009) Risk factors impact on the long-term survival after hemorrhagic stroke. Med. Arch. 63: 203–206.
4. Keep RF, Hua Y, Xi G (2012) Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 11: 720–731.
5. Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF (2001) Spontaneous intracerebral hemorrhage. N. Engl. J. Med. 344: 1450–1460.
6. Øie LR, Madsbu MA, Solheim O, Jakola AS, Giannadakis C, Vorhaug A, Padayachy L, Jensberg H, Dodick D, Salvesen Ø, Gulati S (2018) Functional outcome and survival following spontaneous intracerebral hemorrhage: A retrospective population-based study. Brain Behav. 8: e01113.
7. Brott T, Kothari JR, Barsan W, Tomsick T, Sauerbeck L, Spilker J, Duldner J, Khoury J (1997) Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke 28: 1–5.
8. Mayer SA, Rincon F (2005) Treatment of intracerebral haemorrhage. Lancet Neurol. 4: 662–672.
9. Mayer SA, Sacco RL, Shi T, Mohr JP (1994) Neurologic deterioration in noncomatose patients with supratentorial intracerebral hemorrhage. Neurology 44: 1379–1384.
10. Aronowski J, Zhao X (2011) Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke 42: 1781–1786.
11. Righy C, Bozza MT, Oliveira MF, Bozza FA (2016) Molecular, cellular and clinical aspects of intracerebral hemorrhage: are the enemies within? Curr. Neuropharmacol. 14: 392–402.
12. Huang F-P, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT (2002) Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J. Neurosurg. 96: 287–293.
13. Xi G, Keep RF, Hoff JT (1998) Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J. Neurosurg. 89: 991–996.
14. Hu X, Tao C, Gan Q, Zheng J, Li H, You C (2016) Oxidative stress in intracerebral hemorrhage: sources, mechanisms, and therapeutic targets. Oxid. Med. Cell Longev. 2016: 3215391.
15. Tan DX, Manchester LC, Liu X, Rosales-Corral SA, Acuna-Castroviejo D, Reiter RJ (2013) Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin's primary function and evolution in eukaryotes. J. Pineal Res. 54: 127–138.
16. Suofu Y, Li W, Jean-Alphonse FG, Jia J, Khattar NK, Li J, Baranov SV, Leronni D, Mihalik AC, He Y, Cecon E, Wehbi VL, Kim J, Heath BE, Baranova OV, Wang X, Gable MJ, Kretz ES, Di Benedetto G, Lezon TR, Ferrando LM, Larkin TM, Sullivan M, Yablonska S, Wang J, Minnigh MB, Guillaumet G, Suzenet F, Richardson RM, Poloyac SM, Stolz DB, Jockers R, Witt-Enderby PA, Carlisle DL, Vilardaga JP, Friedlander RM (2017) Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. U.S.A. 114: E7997–E8006.
17. Manchester LC, Poeggeler B, Alvares FL, Ogden GB, Reiter RJ (1995) Melatonin immunoreactivity in the photosynthetic prokaryote Rhodospirillum rubrum: implications for an ancient antioxidant system. Cell Mol. Biol. Res. 41: 391–395.
18. Leung JW, Lau WK, Lau BW, Yee BK (2019) A commentary on the therapeutic potential of melatonin and its analogues in CNS conditions. Psychiatry and Neuroscience Update : From Translational Research to a Humanistic Approach - Volume III, edrs Gargiulo PÁ, Mesones Arroyo HL (Springer International Publishing), pp. 177–186.
19. Stein RM, Kang HJ, McCorvy JD, Glatfelter GC, Jones AJ, Che T, Slocum S, Huang XP, Savych O, Moroz YS, Stauch B, Johansson LC, Cherezov V, Kenakin T, Irwin JJ, Shoichet BK, Roth BL, Dubocovich ML (2000) Virtual discovery of melatonin receptor ligands to modulate circadian rhythms. Nature 579: 609–614.
20. González-Arto M, Aguilar D, Gaspar-Torrubia E, Gallego M, Carvajal-Serna M, Herrera-Marcos LV, Serrano-Blesa E, Hamilton TR, Pérez-Pé R, Muiño-Blanco T, Cebrián-Pérez JA, Casao A (2017) Melatonin MT₁ and MT₂ receptors in the ram reproductive tract. Int. J. Mol. Sci. 18: 662.
21. Masana MI, Doolen S, Ersahin C, Al-Ghoul WM, Duckles SP, Dubocovich ML, Krause DN (2002) MT(2) melatonin receptors are present and functional in rat caudal artery. J. Pharmacol. Exp. Ther. 302: 1295–1302.
22. Carrillo-Vico A, Lardone PJ, Alvarez-Sánchez N, Rodríguez-Rodríguez A, Guerrero JM (2013) Melatonin: buffering the immune system. Int. J. Mol. Sci. 14: 8638–8683.
23. Alghamdi BS (2018) The neuroprotective role of melatonin in neurological disorders. J. Neurosci. Res. 96: 1136–1149.
24. Monteiro MC, Coleman MD, Hill EJ, Prediger RD, Maia CSF (2017) Neuroprotection in neurodegenerative disease: from basic science to clinical applications. Oxid. Med. Cell Longev. 2017: 2949102.
25. Vincent B (2018) Protective roles of melatonin against the amyloid-dependent development of Alzheimer’s disease: a critical review. Pharmacol. Res. 134: 223–237.
26. Leung JW, Cheung K, Ngai SP, Tsang HW, Lau BW (2020) Protective effects of melatonin on neurogenesis impairment in neurological disorders and its relevant molecular mechanisms. Int. J. Mol. Sci. 21: 5645.
27. Andrabi SS, Parvez S, Tabassum H (2015) Melatonin and ischemic stroke: mechanistic roles and action. Adv. Pharmacol. Sci. 2015: 384750 (2015).
28. Macleod MR, O’Collins T, Horky LL, Howells DW, Donnan GA (2005) Systematic review and meta-analysis of the efficacy of melatonin in experimental stroke. J. Pineal Res. 38: 35–41.
29. Reiter RJ, Sainz RM, Lopez-Burillo S, Mayo JC, Manchester LC, Tan DX (2003) Melatonin ameliorates neurologic damage and neurophysiologic deficits in experimental models of stroke. Ann. N. Y. Acad. Sci. 993: 35–47; discussion 48–53.
30. Manaenko A, Chen H, Zhang JH, Tang J (2011) Comparison of different preclinical models of intracerebral hemorrhage. Acta Neurochir. Suppl. 111: 9–14.
31. Xu W, Lu X, Zheng J, Li T, Gao L, Lenahan C, Shao A, Zhang J, Yu J (2018) Melatonin protects against neuronal apoptosis via suppression of the ATF6/CHOP pathway in a rat model of intracerebral hemorrhage. Front. Neurosci. 12: 638.
32. Wang Z, Zhou F, Dou Y, Tian X, Liu C, Li H, Shen H, Chen G (2018) Melatonin alleviates intracerebral hemorrhage-induced secondary brain injury in rats via suppressing apoptosis, inflammation, oxidative stress, DNA damage, and mitochondria injury. Transl. Stroke Res. 9: 74–91.
33. Li Z, Liang G, Xue Y, Liu Y (2009) Effects of combination treatment of dexamethasone and melatonin on brain injury in intracerebral hemorrhage model in rats. Brain Res. 1264: 98–103.
34. MacLellan CL, Silasi G, Auriat AM, Colbourne F (2010) Rodent models of intracerebral hemorrhage. Stroke 41: S95–98.
35. Hartman RE, Rojas HA, Lekic T, Ayer R, Lee S, Jadhav V, Titova E, Tang J, Zhang JH (2008) Long-term effects of melatonin after intracerebral hemorrhage in rats. Acta Neurochir. Suppl. 105: 99–100.
36. Rojas H, Lekic T, Chen W, Jadhav V, Titova E, Martin RD, Tang J, Zhang J (2008) The antioxidant effects of melatonin after intracerebral hemorrhage in rats. Acta Neurochir. Suppl. 105: 19–21.
37. Lekic T, Hartman R, Rojas H, Manaenko A, Chen W, Ayer R, Tang J, Zhang JH (2010) Protective effect of melatonin upon neuropathology, striatal function, and memory ability after intracerebral hemorrhage in rats. J. Neurotrauma 27: 627–637.
38. Sang YH, Liang YX, Liu LG, Ellis-Behnke RG, Wu WT, So KF, Cheung RT (2013) Rat model of intracerebral hemorrhage permitting hematoma aspiration plus intralesional injection. Exp. Anim. 62: 63–69.
39. Sang LY, Liang YX, Li Y, Wong WM, Tay DK, So KF, Ellis-Behnke RG, Wu W, Cheung RT. (2015) A self-assembling nanomaterial reduces acute brain injury and enhances functional recovery in a rat model of intracerebral hemorrhage. Nanomedicine 11: 611–620.
40. Sang YH, Su HX, Wu WT, So KF, Cheung RT (2011) Elevated blood pressure aggravates intracerebral hemorrhage-induced brain injury. J. Neurotrauma 28: 2523–2534.
41. Liu L, Cheung RT (2013) Effects of pretreatment with a combination of melatonin and electroacupuncture in a rat model of transient focal cerebral ischemia. Evid. Based Complement. Alternat. Med. 2013: 953162.
42. Yang Z, Liu B, Zhong L, Shen H, Lin C, Lin L, Zhang N, Yuan B (2015) Toll-like receptor-4-mediated autophagy contributes to microglial activation and inflammatory injury in mouse models of intracerebral haemorrhage. Neuropathol. Appl. Neurobiol. 41: e95–e106.
43. Zhao X, Song S, Sun G, Strong R, Zhang J, Grotta JC, Aronowski J (2009) Neuroprotective role of haptoglobin after intracerebral hemorrhage. J. Neurosci. 29: 15819–15827.
44. Mracsko E, Veltkamp R (2014) Neuroinflammation after intracerebral hemorrhage. Front. Cell. Neurosci. 8: 388.
45. Duan X, Wen Z, Shen H, Shen M, Chen G (2016) Intracerebral hemorrhage, oxidative stress, and antioxidant therapy. Oxid. Med. Cell Longev. 2016: 1203285.
46. Tan DX, Manchester LC, Esteban-Zubero E, Zhou Z, Reiter RJ (1995) Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules 20: 18886-18906.
47. Chen X, Xi Z, Liang H, Sun Y, Zhong Z, Wang B, Bian L, Sun Q (2019) Melatonin prevents mice cortical astrocytes from hemin-induced toxicity through activating PKCα/Nrf2/HO-1 signaling in vitro. Front. Neurosci. 13: 760.
48. Tang J, Chen R, Wang L, Yu L, Zuo D, Cui G, Gong X (2020) Melatonin attenuates thrombin-induced inflammation in BV2 cells and then protects HT22 cells from apoptosis. Inflammation 43: 1959–1970.
49. Wang J (2010) Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog. Neurobiol. 92: 463–477.
50. Wang J, Doré S (2007) Inflammation after intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 27: 894–908.
51. Taylor RA, Sansing LH (2013) Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin. Dev. Immunol. 2013: 746068.
52. Lan X, Han X, Li Q, Yang Q-W, Wang J (2017) Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat. Rev. Neurol. 13: 420–433.
53. Del Bigio MR, Yan HJ, Buist R, Peeling J (1996) Experimental intracerebral hemorrhage in rats. Magnetic resonance imaging and histopathological correlates. Stroke 27: 2312–2319; discussion 2319–2320.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


