Molecular mechanisms of melatonin’s protection against high-LET radiation: implications for space travel
Melatonin protection against high-LET radiation
Abstract
During a deep space mission, the central nervous system (CNS) and other organs are exposed to galactic cosmic rays and solar particle events. Health risks associated with various organs and systems are important issues in a long-term spaceflight. Potential CNS damage during a space mission could alter cognitive functions which might impact performance and individual’s health. The neuronal injury originating from exposure to 56Fe particle irradiation involves the elevated oxidative stress which can be inhibited by melatonin pretreatment. Melatonin exerts potent neuroprotective effects against carbon ion-induced mitochondrial dysfunction and apoptosis in the mouse brain. A significant increase in the count of immature neurons and proliferating cells was detected in the mice under 56Fe particle irradiation cotreated with the melatonin metabolite, AFMK. Melatonin treatment also significantly reduced the carbon ion-induced apoptotic cells and elevated oxidative stress in the mouse testis. The results suggest that melatonin treatment is a potential strategy to protect against space radiation hazards. Spaceflight-induced molecular, cellular, and physiologic changes lead to alterations across many organs and systems. Epigenetic, gene expression, inflammatory, and metabolic responses to spaceflight should be examined and means to safe-guard against these changes in upcoming missions. Precision medicine will be crucial for assessing and augmenting efficacy of melatonin or other medications in astronauts. In addition, enhancing radio-resistance of humans is a novel strategy for a long-term space mission. Further investigations with a combination of melatonin and other novel technologies are warranted to better alleviate HZE particle irradiation-induced damage to astronauts on long-term space exploration missions.
References
2. Simpson JA (1983) Elemental and isotopic composition of the galactic cosmic rays. Ann. Rev. Nucl. Part. Sci. 33: 323-381.
3. Maalouf M, Durante M, Foray N (2011) Biological effects of space radiation on human cells: history, advances and outcomes. J. Radiat. Res. 52: 126-146.
4. Nelson GA (2016) Space radiation and human exposures, a primer. Radiat. Res. 185: 349-358.
5. Kim MH, Wilson JW, Cucinotta FA (2012) Description of transport codes for space radiation shielding. Health Phys. 103: 621-639.
6. Chancellor JC, Scott GB, Sutton JP (2014) Space radiation: The number one risk to astronaut health beyond low Earth orbit. Life (Basel) 4: 491-510.
7. Guipaud O, Jaillet C, Clément-Colmou K, et al. (2018) The importance of the vascular endothelial barrier in the immune-inflammatory response induced by radiotherapy. Br. J. Radiol. 91: 20170762.
8. Schaue D, Kachikwu EL, McBride WH (2012) Cytokines in radiobiological responses: a review. Radiat. Res. 178: 505-523.
9. Werner E, Wang H, Doetsch PW (2015) Role of pro-inflammatory cytokines in radiation-induced genomic instability in human bronchial epithelial cells. Radiat. Res. 184: 621-629.
10. Schaue D, McBride WH (2012) T lymphocytes and normal tissue responses to radiation. Front. Oncol. 2: 119.
11. Wirsdörfer F, Jendrossek V (2016) The role of lymphocytes in radiotherapy-induced adverse late effects in the lung. Front. Immunol. 7: 591.
12. Reiter RJ, Tan DX, Korkmaz A, et al. (2011) The disaster in Japan: utility of melatonin in providing protection against ionizing radiation. J. Pineal Res. 50: 357-358.
13. Reiter RJ, Rosales-Corral S, Tan DX, et al. (2017) Melatonin as a mitochondria-targeted antioxidant: one of evolution's best ideas. Cell Mol. Life Sci. 74: 3863-3881.
14. Reiter RJ, Tan DX, Rosales-Corral S, et al. (2018) Mitochondria: central organelles for melatonin's antioxidant and anti-aging actions. Molecules 23: 509-533.
15. Manchester LC, Coto-Montes A, Boga JA, et al. (2015) Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 59: 403-419.
16. Reiter RJ, Ma Q, Sharma R (2020) Melatonin in mitochondria: Mitigating clear and present dangers. Physiology (Bethesda) 35: 86-95.
17. Kouhi Habibi N, Shabestani Monfared A, Ebrahimnejad Gorji K, et al. (2019) The protective effects of melatonin on blood cell counts of rectal cancer patients following radio-chemotherapy: a randomized controlled trial. Clin. Transl. Oncol. 21: 745-752.
18. Vijayalaxmi, Reiter RJ, Meltz ML (1995) Melatonin protects human blood lymphocytes from radiation-induced chromosome damage. Mutat. Res. 346: 23-31.
19. Vijayalaxmi, Reiter RJ, Herman TS, et al. (1996) Melatonin and radioprotection from genetic damage: in vivo/in vitro studies with human volunteers. Mutat. Res. 37: 221-228.
20. Vijayalaxmi, Reiter RJ, Herman TS, et al. (1998) Melatonin reduces gamma-radiation-induced primary DNA damage in human blood lymphocytes. Mutat. Res. 347: 203-208.
21. Vijayalaxmi, Meltz ML, Reiter RJ, et al. (1999) Melatonin and protection from whole-body irradiation: survival studies in mice. Mutat. Res. 425: 21-27.
22. Vijayalaxmi, Meltz ML, Reiter RJ, et al. (1999) Melatonin and protection from genetic damage in blood and bone marrow: whole-body irradiation studies in mice. J. Pineal Res. 27: 221-225.
23. Musa AE, Shabeeb D, Alhilfi HSQ (2019) Protective effect of melatonin against radiotherapy-induced small intestinal oxidative stress: biochemical evaluation. Medicina (Kaunas). 55: 308-318.
24. Shabeeb D, Najafi M, Musa AE, et al. (2019) Biochemical and histopathological evaluation of the radioprotective effects of melatonin against gamma ray-induced skin damage. Curr. Radiopharm. 12: 72-81.
25. Korkmaz A, Tan DX, Reiter RJ (2011) Melatonin: an established radioprotective agent against Japan’s nuclear disaster. TAF Prev. Med. Bull. 10: 127-129.
26. Tarocco A, Caroccia N, Morciano G. et al. (2019) Melatonin as a master regulator of cell death and inflammation: molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 10: 317-328.
27. Rango M, Bresolin N (2018) Brain mitochondria, aging, and Parkinson's disease. Genes (Basel). 9: 250.
28. Livingston K, Schlaak RA, Puckett LL, et al. (2020) The role of mitochondrial dysfunction in radiation-induced heart disease: from bench to bedside. Front. Cardiovasc. Med. 7:20.
29. Rogounovitch TI, Saenko VA, Shimizu-Yoshida Y, et al. (2002) Large deletions in mitochondrial DNA in radiation-associated human thyroid tumors. Cancer Res. 62: 7031-7041.
30. Wilding CS, Cadwell K, Tawn EJ, et al. (2006) Mitochondrial DNA mutations in individuals occupationally exposed to ionizing radiation. Radiat. Res. 165: 202-207.
31. Tan DX, Reiter RJ (2019) Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2: 44-66.
32. Mao XW, Boerma M, Rodriguez D, et al. (2018) Acute effect of low-dose space radiation on mouse retina and retinal endothelial cells. Radiat. Res. 190: 45-52.
33. Zhang Y, Wang Q, Chen H, et al. (2018) Involvement of cholinergic dysfunction and oxidative damage in the effects of simulated weightlessness on learning and memory in rats. Biomed. Res. Int. 2018: 2547532.
34. Majidinia M, Reiter RJ, Shakouri SK, et al. (2018) The role of melatonin, a multitasking molecule, in retarding the processes of ageing. Ageing Res. Rev. 47: 198-213.
35. Dong J, Sulik KK, Chen SY (2008) Nrf2-mediated transcriptional induction of antioxidant response in mouse embryos exposed to ethanol in vivo: implications for the prevention of fetal alcohol spectrum disorders. Antioxid. Redox Signal 10: 2023-2033.
36. Ma YF, Wu ZH, Gao M, et al. (2018) Nuclear factor erythroid 2-related factor 2 antioxidant response element pathways protect bovine mammary epithelial cells against H2O2-induced oxidative damage in vitro. J. Dairy Sci. 101: 5329‐5344.
37. Raghunath A, Sundarraj K, Nagarajan R, et al. (2018) Antioxidant response elements: Discovery, classes, regulation and potential applications. Redox Biol. 17: 297-314.
38. Tonelli C, Chio IIC, Tuveson DA (2018) Transcriptional regulation by Nrf2. Antioxid. Redox Signal 29 :1727-1745.
39. Liu Y, Zhang L, Zhang H, et al. (2012) Exogenous melatonin modulates apoptosis in the mouse brain induced by high-LET carbon ion irradiation. J. Pineal Res. 52: 47-56.
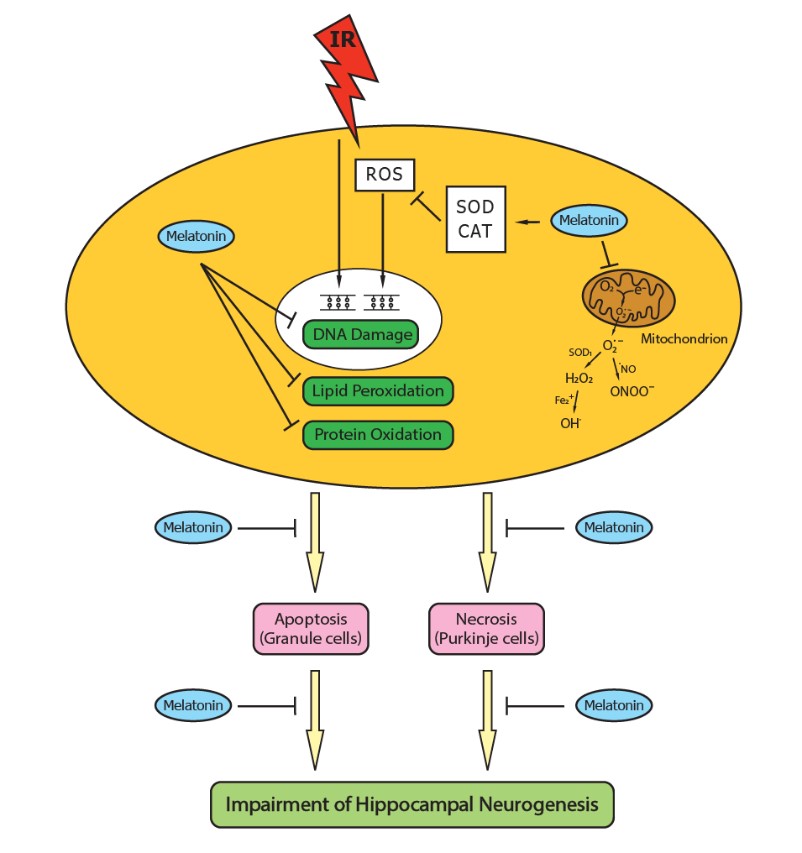
40. Manda K, Ueno M, Anzai K (2008) Melatonin mitigates oxidative damage and apoptosis in mouse cerebellum induced by high-LET 56Fe particle irradiation. J. Pineal Res. 44: 189-196.
41. Xiao M, Zhong H, Xia L, et al. (2017) Pathophysiology of mitochondrial lipid oxidation: Role of 4-hydroxynonenal (4-HNE) and other bioactive lipids in mitochondria. Free Radic. Biol. Med. 111: 316-327.
42. Sousa BC, Pitt AR, Spickett CM (2017) Chemistry and analysis of HNE and other prominent carbonyl-containing lipid oxidation compounds. Free Radic. Biol. Med. 111: 294-308.
43. Manda K, Ueno M, Anzai K (2008) Space radiation-induced inhibition of neurogenesis in the hippocampal dentate gyrus and memory impairment in mice: ameliorative potential of the melatonin metabolite, AFMK. J. Pineal Res. 45: 430-438.
44. Tseng BP, Giedzinski E, Izadi A, et al. (2014) Functional consequences of radiation-induced oxidative stress in cultured neural stem cells and the brain exposed to charged particle irradiation. Antioxid. Redox Signal 20: 1410-1422.
45. Mishra B, Luderer U (2019) Reproductive hazards of space travel in women and men. Nat. Rev. Endocrinol. 15: 713-730.
46. Liu Y, Zhang H, Zhang L, et al. (2009) Melatonin modulates acute testicular damage induced by carbon ions in mice. Pharmazie 64: 685-689.
47. Cucinotta FA, Cacao E (2019) Risks of cognitive detriments after low dose heavy ion and proton exposures. Int. J. Radiat. Biol. 95: 985-998.
48. Cucinotta FA, Alp M, Sulzman FM, et al. (2014) Space radiation risks to the central nervous system. Life Sci. Space Res. 2: 54-69.
49. McLaughlin MF, Donoviel DB, Jones JA (2017) Novel indications for commonly used medications as radiation protectants in spaceflight. Aerosp. Med. Hum. Perform. 88: 665-676.
50. Iosim S, MacKay M, Westover C, et al. (2019) Translating current biomedical therapies for long duration, deep space missions. Precis. Clin. Med. 2: 259-269.
51. Furukawa S, Nagamatsu A, Nenoi M, et al. (2020) Space radiation biology for "Living in Space". Biomed. Res. Int. 2020: 4703286.
52. Cortese F, Klokov D, Osipov A, et al. (2018) Vive la radiorésistance!: converging research in radiobiology and biogerontology to enhance human radioresistance for deep space exploration and colonization. Oncotarget. 9:14692-14722.
53. Hashimoto T, Horikawa DD, Saito Y, et al. (2016) Extremotolerant tardigrade genome and improved radiotolerance of human cultured cells by tardigrade-unique protein. Nat. Commun. 7:12808.
54. Chavez C, Cruz-Becerra G, Fei J, et al. (2019) The tardigrade damage suppressor protein binds to nucleosomes and protects DNA from hydroxyl radicals. Elife. 8: e47682.
55. Tan DX, Chen LD, Poeggeler B, et al. (1993) Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr. J. 1: 57-60.

This work is licensed under a Creative Commons Attribution 4.0 International License.
For all articles published in Melatonin Res., copyright is retained by the authors. Articles are licensed under an open access Creative Commons CC BY 4.0 license, meaning that anyone may download and read the paper for free. In addition, the article may be reused and quoted provided that the original published version is cited. These conditions allow for maximum use and exposure of the work, while ensuring that the authors receive proper credit.
In exceptional circumstances articles may be licensed differently. If you have specific condition (such as one linked to funding) that does not allow this license, please mention this to the editorial office of the journal at submission. Exceptions will be granted at the discretion of the publisher.


