Zimmerman, S. and Reiter, R.J. 2019. Melatonin and the Optics of the Human Body. Melatonin Research. 2, 1 (Feb. 2019) 138-160. DOI:https://doi.org/https://doi.org/10.32794/mr11250016.
Review
Melatonin and the Optics of the Human Body
Scott Zimmerman*1 and Russel J. Reiter2
1Silas Inc., Basking Ridge, New Jersey, 07920, USA
2Department of Cell Systems and Anatomy UT Health Science Center San Antonio, Texas, 78229 USA
* correspondence: scott.zimmmerman@silas.tech; Tel: +01-9732707414
Running title: Melatonin and the Optics
Received: January 12, 2019; Accepted: February 21, 2019
ABSTRACT
Melatonin is fundamental to the lighting, display, and architectural industries as the primary biomarker used in circadian theory. Billions of dollars are being spent on research, product development, and marketing based on the impact of visible light on melatonin produced by the pineal gland. It has now been shown that the mitochondria produce melatonin in many cells in quantities which are orders of magnitude higher than that produced in the pineal gland. This subcellular melatonin does not necessarily fluctuate with our circadian clock or release into the circulation system, but instead has been proposed to be consumed locally in response to the free radical density within each cell, in particular in response to Near Infrared (NIR) exposure. The main point of this review hypothesizes that the subcellular melatonin is being produced in response to the NIR photons which make up the majority of natural sunlight. Given the number of cells and quantity of subcellular melatonin identified to date, it is reasonable to propose that the body produces and maintains a melatonin reservoir that is separate and apart from the circulatory melatonin generated by the pineal gland. To understand how sunlight may support or stimulate this antioxidant reservoir, it becomes necessary to quantify the free radical density in various parts of the human body. To do this, it is necessary to move away from two-dimensional empirical approaches and develop three-dimensional bio-optical models based on the underlying biological processes at play. Three-dimensional Mechanistic Bio-optical Models (MBM) of the skin, eye, and brain based on non-sequential optical ray tracing and Electron Spin Resonance (ESR) data clearly indicate that the NIR portion of natural sunlight provides the primary stimulus during the day to the majority of the cells in the human body, impacting over 60% of the cells in an adult body and 100% of the cells in the fetus and young children. It is also shown that optically, the human body, under the assumption of natural sunlight, has developed optical mechanisms to gather and localize NIR photons in the most sensitive areas of the human body: blood vessels, retina, brain, skin, and even the fetus. That assumption is no longer valid in modern societies where the majority of our time is spent exposed to visible only lighting and displays, which emit zero NIR photons. Based on an optical and biological review of the literature and the MBM results, it is proposed that the NIR portion of natural sunlight stimulates an excess of antioxidants in each of our healthy cells and that the cumulative effect of this antioxidant reservoir is to enhance the body’s ability to rapidly and locally deal with changing conditions throughout the day. In this approach the role of circulatory melatonin produced by the pineal gland is to provide an efficient method of delivering supplemental melatonin during periods of low cellular activity and solar stimulus to damaged or aging cells in both diurnal and nocturnal animals. While circulatory melatonin may be the “Hormone of Darkness”, subcellular melatonin may be the “Hormone of Daylight”.
Keywords: circulatory melatonin, subcellular melatonin, circadian, free radical, Electron Spin Resonance, LED lighting, sunlight, optical ray tracing.
__________________________________________________________________________________________
1. INTRODUCTION
As proposed by Tan et al (1) and subsequently confirmed, melatonin is produced and has been detected in the mitochondria in many cells and has also been identified in extrapineal tissues including the brain, retina, lens, cochlea, Harderian gland, airway epithelium, skin, gastrointestinal tract, liver, kidney, thyroid, pancreas, thymus, spleen, immune system cells, carotid body, reproductive tract and endothelial cells in quantities which can be orders of magnitude higher the circulatory melatonin produced by the pineal gland. The hypothesis of this review is that unlike circulatory melatonin generated in the pineal gland, subcellular melatonin (along with other antioxidants) is produced in the mitochondria as a local response to the local free radical density within most of our cells. Biosynthesis of melatonin has been demonstrated in mitochondria of different species and a variety of types of cells by researchers (2-4). Odinokov and Hamblin (5) have proposed that NIR photons stimulate subcellular or extrapineal melatonin biosynthesis via cyclic adenosine monophosphate (AMP) or NF-kB activation, or alternatively by stimulating bone marrow stem cells. In general, the formation of free radicals via NIR absorption in various chromophores is the action mechanism which forms the basis of NIR phototherapy treatments for wound healing, dementia, macular degeneration, and PTSD (6-9). Melatonin, as well as its metabolites, are known to be highly effective direct free radical scavengers (10) and can also function as indirect antioxidants by stimulating other antioxidant enzymes (11). It has been reported that subcellular melatonin exists in many tissues at higher concentration levels than circulatory melatonin in the blood (12-14). Unlike circulatory melatonin (endocrine), subcellular melatonin appears to be produced and stay primarily near the cell in which it is generated (autocrine/paracrine). As will be discussed further below, at the cellular level, sunlight, ATP production, etc. are generating high free radical densities at rates which indicate the need for an immediate local antioxidant response. At a cellular level, it is reasonable to assert that free radicals must be neutralized via antioxidants at a rate sufficient to maintain cellular health and prevent molecular debris accumulation (15). In this scenario, healthy cells typically contain an excess of antioxidants.
∑ 𝐴𝑛𝑡𝑖𝑜𝑥𝑖𝑑𝑎𝑛𝑡𝑠 = 𝛼 ∑ 𝐹𝑟𝑒𝑒 𝑅𝑎𝑑𝑖𝑐𝑎𝑙𝑠
𝑤ℎ𝑒𝑟𝑒 𝛼 > 1
Based on this conjecture, each healthy cell would be constantly refilling its own antioxidant stores in response to the number of free radicals generated by energy production, sunlight, pathogens, etc. During the day, high cellular activity and exposure to sunlight will require antioxidant responses with time constants in the range of seconds as internal and external conditions in each cell change. It is proposed that the cumulative quality of these antioxidant reservoirs may explain why red/NIR phototherapies have been shown to improve how well we sleep, how much circulatory melatonin is extracted from the blood during sleep, and how well we function the following day (16).
This hypothesis unlike existing circadian theory is also consistent with nocturnal animals producing high circulatory melatonin during the night. It is accepted that the SCN signals the release of circulatory melatonin from the pineal gland in response to absence of blue and green photons to the retina. The fact that nocturnal animals and night shift workers continue to produce high levels of circulatory melatonin during periods of high cellular activity (17) appears to indicate melatonin’s antioxidant properties are of primary importance in mammals.
As antioxidants and free radicals battle, molecular debris is generated. The accumulation of debris from this constant battle is known to occur and has been linked to aging (18). As such, subcellular melatonin may be a better biomarker for cellular health than circulatory melatonin. It is also proposed that the pineal gland’s primary function is to provide supplemental melatonin (circulatory melatonin) during periods of low cellular activity in diurnal creatures using the chemical concentration gradient across the cell membrane to efficiently deliver melatonin to only those cells which need the supplement the most. This is in addition to providing circadian information. Based on this perspective, if the number of free radicals generated in each of cell was known, the antioxidant response could be estimated along with the likelihood of debris accumulation. The photon absorption profile in each wavelength range can be calculated via non-sequential optical ray traces which in turn leads to the three-dimensional free radical distribution using Electron Spin Resonance data. From these distributions an estimate of the three-dimensional antioxidant distribution required by healthy cells can be calculated. Estimates of molecular debris/inflammation accumulation can also be calculated. Given that virtually all the research in this field has been based on two-dimensional empirical data, a three-dimensional mechanistic approach provides significant insight into some the body’s most basic biological processes. In order to proceed, it is necessary to first look at the problem from an optics perspective.
2. OPTICS OF THE HUMAN BODY
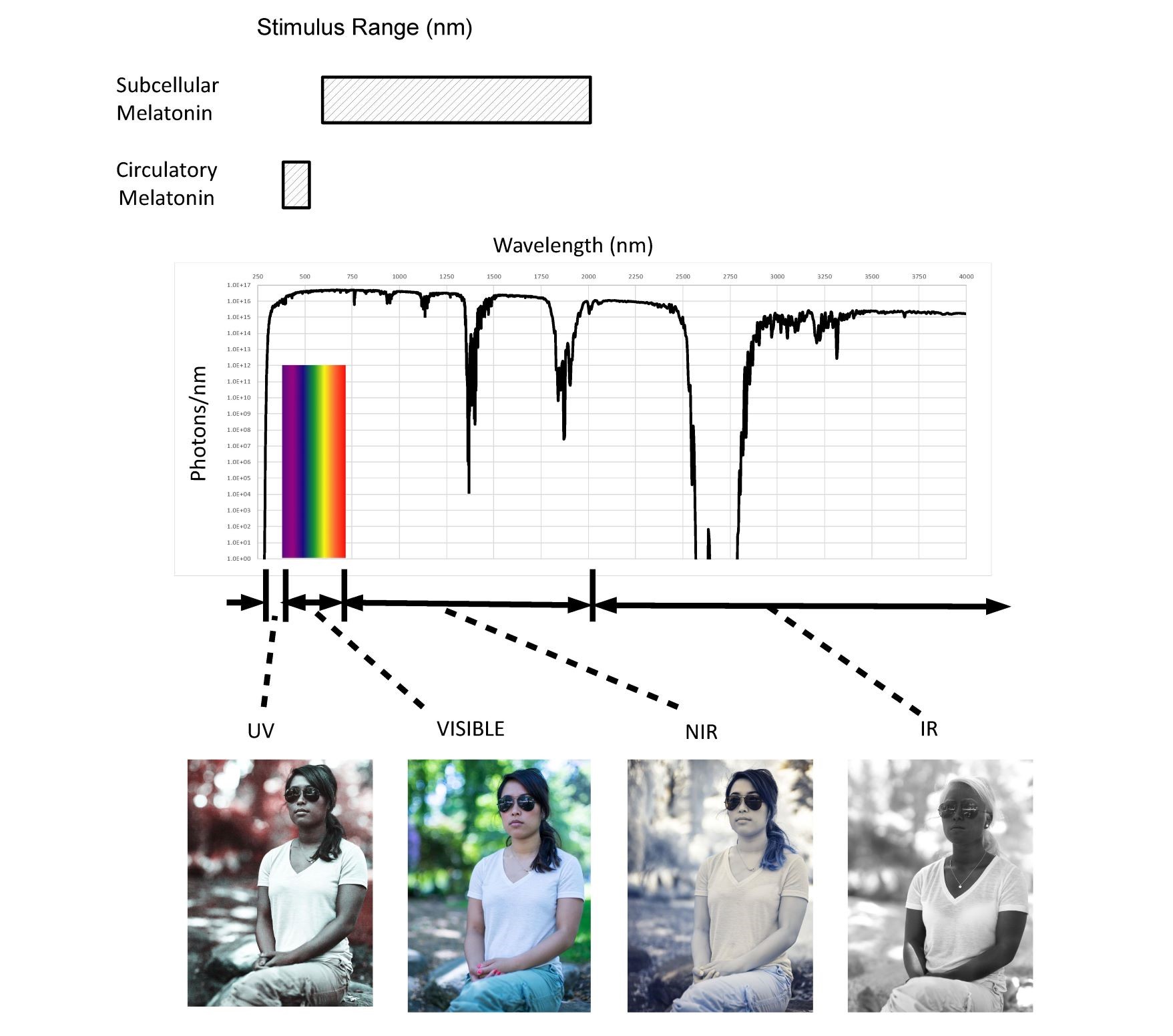
Sunlight represents for millions of years the single largest external stimulus to the human body (up to 60 MJ/day). As shown in Figure 1, the sun is a broadband predominately NIR emitter exposing us to photons with wavelengths ranging from 250nm to greater than 4000nm.

Fig. 1 Solar spectrum (250-4,000 nm) and proposed stimulus ranges
The active range for circulatory melatonin extends from 420nm to 500nm. It is proposed that subcellular melatonin is stimulated by NIR photons from 650nm to 1200nm. NIR represents 70% of the total solar spectrum from a photochemistry viewpoint (photons/second). The pictures illustrate the broad range of optical properties that the human body exhibits throughout the solar spectral range from 250nm to over 4000nm.
There has been limited research into the optics of the human body. As shown in the pictures provided with permission from Nick Spiker, both absorption and scatter coefficients vary significantly with wavelength. Provided by Bashkatov et al (19), optical coefficients for many of the body’s tissues are available making it possible to model the eye, skin, brain, fetus, etc.
As shown, circulatory melatonin levels and existing circadian rhythms depend on only approximately 2% of the spectrum emitted by the Sun. According to chronobiologists the absence of exposure of the retina to violet, blue, and green photons trigger the SCN to stimulate the pineal gland to secrete circulatory melatonin (20). This narrow range of wavelengths (violet, blue and green) corresponds to the blue of the sky. As such, modern Visible Only lighting (VOL) systems are being designed now to have high blue content during the day to make people more alert (suppress circulatory melatonin) and low blue content at night for better sleep (not suppress circulatory melatonin). Based on the optical penetration depth of photons in the NIR, the majority of cells in the human body only “see” NIR photons in what is termed the “therapeutic or biological window”. In natural sunlight over 70% of the photons impinging on the body are NIR photons. LED, OLED, and CFL lighting and displays emit zero NIR photons, thus they can be called “Visible Only” emitters in contrast to sunlight, fire, moonlight, and incandescent sources which emit mostly NIR photons.
Our bodies are not adapted to VOL as shown in Figure 2 and, from a dosage or photochemistry perspective, there is no equivalence between the spectrums emitted by VOL and displays and what we are exposed to in nature.
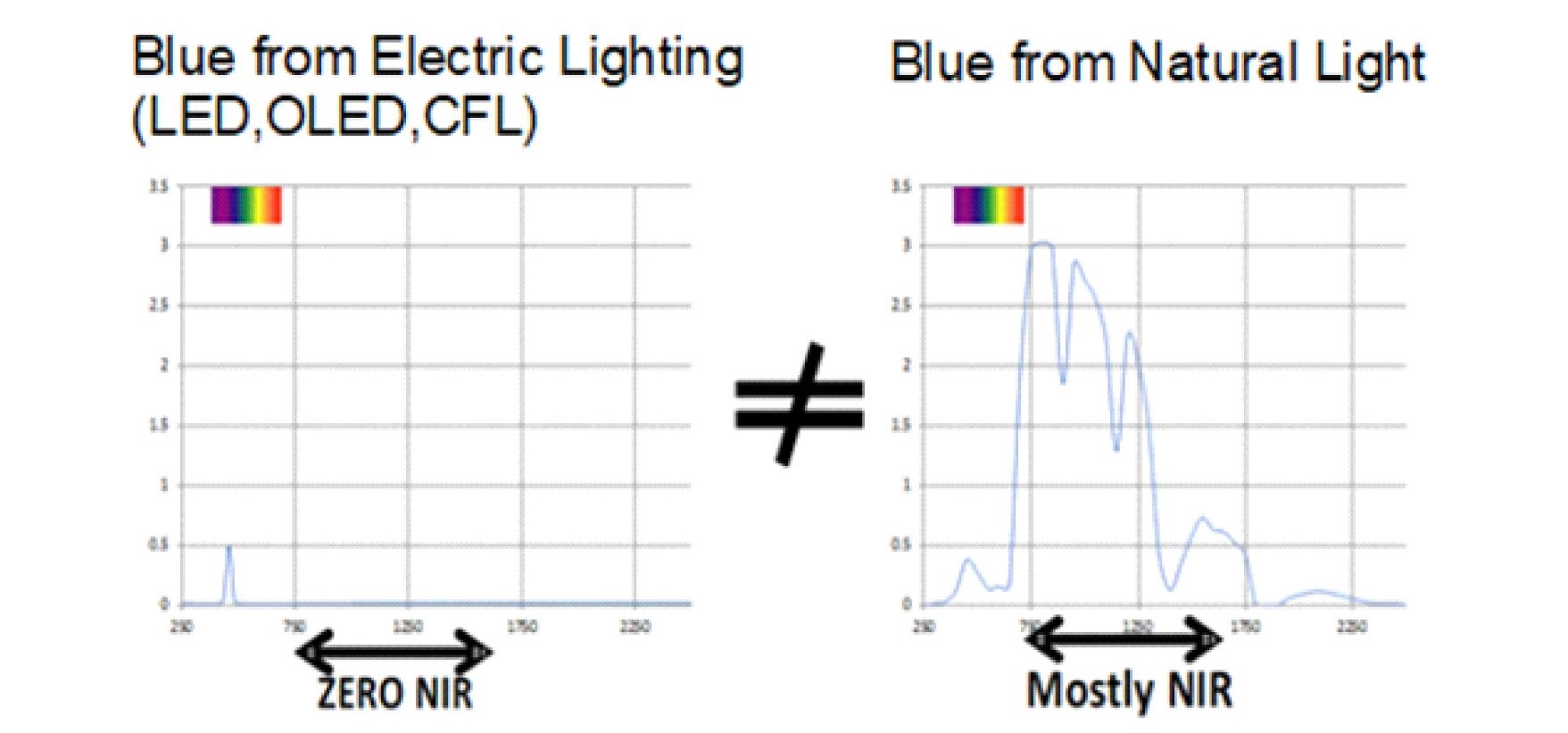
Fig. 2 A comparison between artificial blue light and blue light in nature.
LED, CFL, OLED lighting and display sources emit zero NIR. The Sun is a predominately NIR emitter this coupled with the reflectance of our surroundings guarantees that our cells are always exposed to an excess of NIR photons compared to UV or Visible photons.
In nature, the colors we see with our eyes are created by strongly absorbing visible photons; however, virtually all of our surroundings strongly scatter NIR photons. As shown in Figure S1, it is incorrect to simply use just the spectrum of the Sun when comparing Visible Only emitters to natural sunlight. The grass, clouds, and even dirt absorb visible photons and strongly reflect NIR photons. Given the corrected spectrum of the source, it becomes possible to model where photons at each wavelength are absorbed are shown in Figure 3.
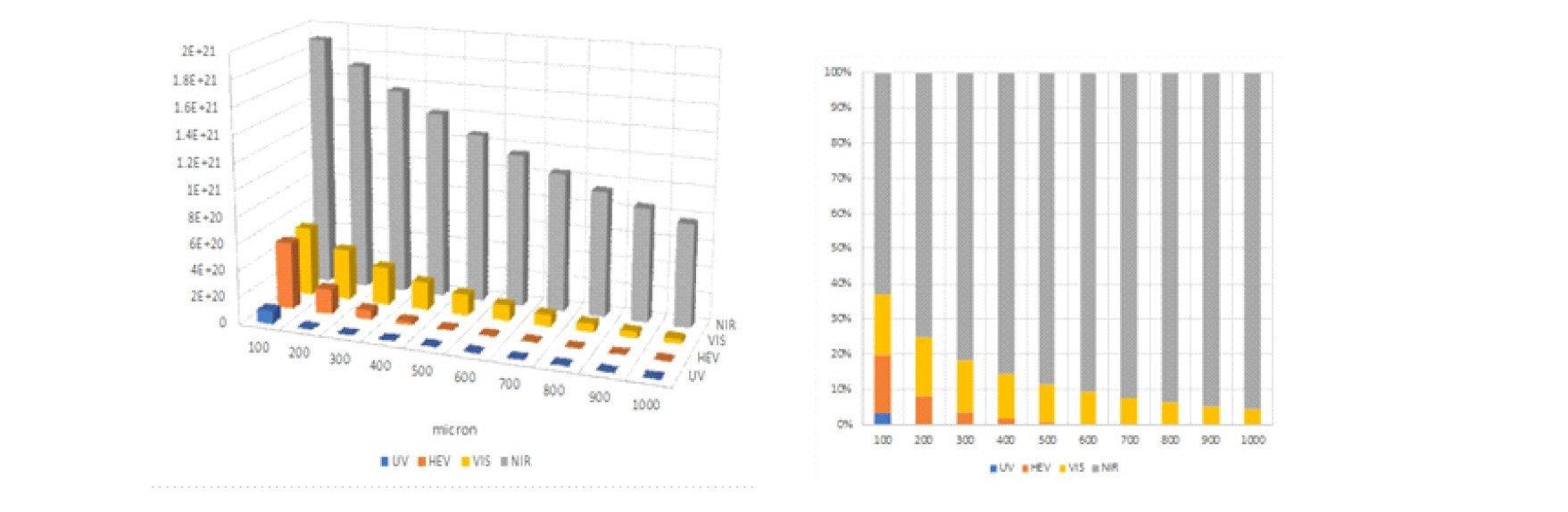
Fig. 3 The number of photons absorbed at various skin depths (8 hours natural sunlight)
The number of absorbed photons in 100 micron slices for UV (250-400nm), HEV (400-500nm), VIS (500-650nm), NIR (650-1200nm) and the relative percentage are plotted.
It is immediately evident that more NIR photons are absorbed under natural sunlight because of the increased number of NIR photons and the NIR biological windows that exist in the human body. It should also be noted the majority of the cells in which UV and HEV photons are absorbed have relative short life cycles and it appears that the human body has chosen to replace rather than repair damage created by these higher energy photons. For sensitive areas like the retina, the models show that the body goes to great lengths to filter out UV, attenuate VIS, and gather NIR photons.
NIR photons are scattered several inches into the body even through the skull due to the low optical absorption and the anisotropic nature of the scatter in the human body in this wavelength range. This leads to 100% of the cells of the fetus and young children being exposed to larger amounts of NIR photons as compared to approximately 60% of the cells in adults as illustrated in Figure 4.
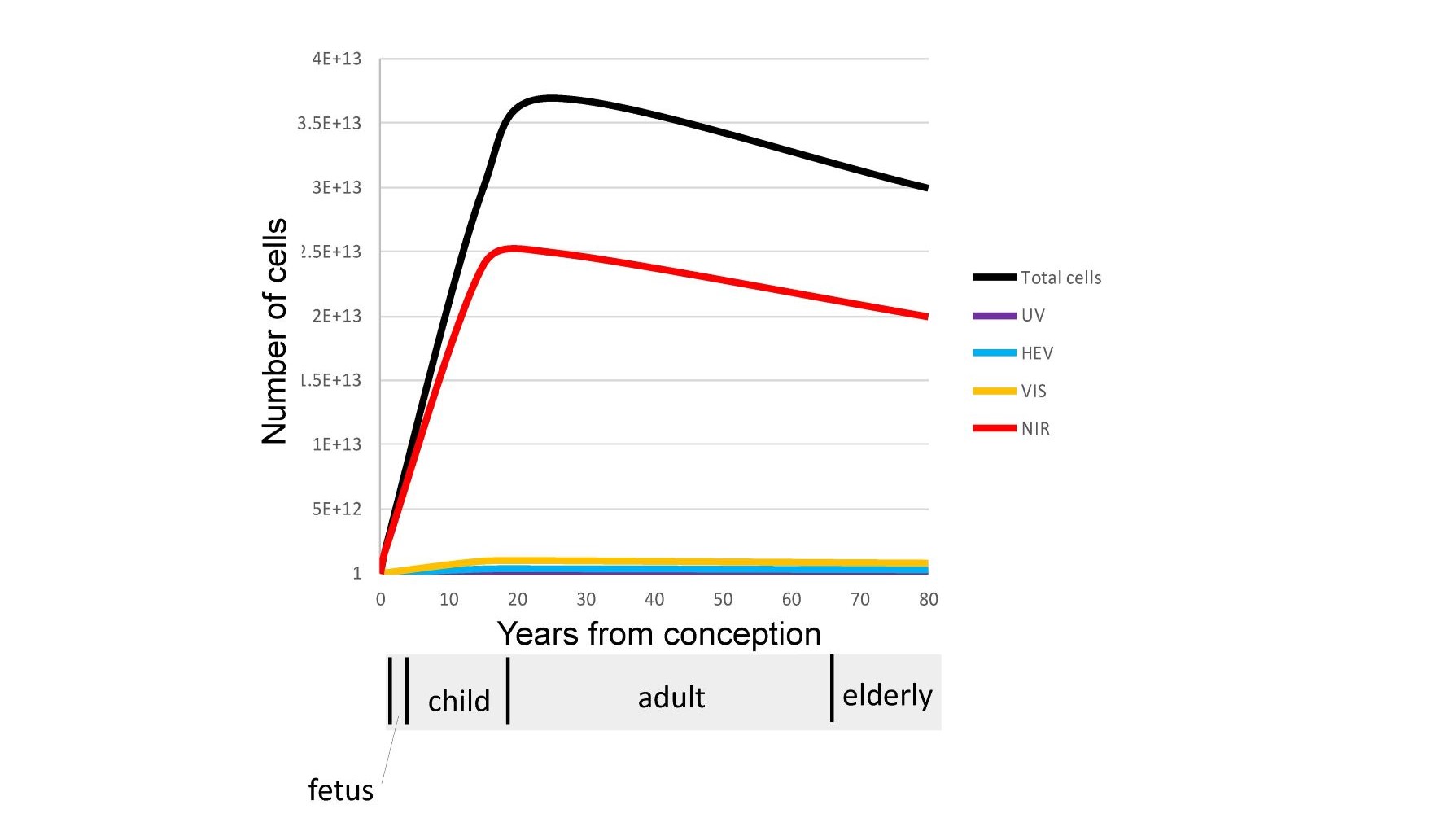
Fig. 4 A comparison of the number of cells in the human body under the influence of UV, HEV, VIS, and NIR wavelengths.
The Biological windows that exist in the NIR result in the majority of our cells only being exposed to NIR photons. Shorter higher energy photons (UV, HEV, VIS) are localized optically in the outer surfaces of our skin and eyes.
To understand how this occurs it is necessary to look at the difference between how photons are transmitted in transparent and translucent materials. The characterization of even thin layers of translucent materials represents one of the most difficult measurements in optics due to the non-Hamiltonian nature of scatter. Unfortunately, the lack of understanding how to properly measure translucent materials has led to a number of incorrect optical measurements in the literature and an underestimation of how far into the body photons of all wavelengths can penetrate. As will be discussed, the human body is a non-homogenous media whose optical properties are strongly wavelength dependent exposed to sources that vary greatly especially in this modern age of artificial lighting and displays.
As shown in Figure 5A translucent materials cannot be accurately measured using the standard source/detector methods as discussed by Tirpak and Young (21) typically used for transparent materials due to the detector missing the majority of the scattered photons. Even the double beam method cited in the reference is limited to thin homogeneous translucent materials due to light losses at sample edges. Instead the use of optical models and imaging techniques are required to correctly estimate the distribution of photons in complex scattering non-homogeneous media like the human body. Also shown in Figure 5A is a comparison between the transmission through the hand of 6 year-old versus a 60 year-old using the same white LED light source in the palm. Given that the optical absorption coefficient in the red region is much larger than in the NIR this effect will be even more pronounced in the NIR.
As shown in Figure 5B the brain provides an excellent example of both the elegance and complexity of the optics of the human body and why the overly simplistic measurement techniques used in the literature are misleading at best. Optically the cerebrospinal (CSF) fluid surrounding the brain has its minimum optical absorption and scatter in the NIR, forming a very efficient substantially transparent light guide encompassed by scattering surfaces. As shown in Figure 5B cut-away, optically the scalp and skull block UV/Visible photons but transmits NIR photons into this substantially transparent region surrounding the brain. The CSF surrounding the brain optically acts as a light guide distributing the NIR photons scattering off the surface of the brain even deep into the folds of the brain. The use of transparent optical light guides coated with white scattering paint has been used for decades in most bezels and faceplates in the automotive and avionics industry to efficiently and uniformly distribute photons from localized sources into large areas in exactly the same manner. As such the brain appears to be optically designed to distribute NIR photons to the grey matter even down into the folds of the brain as illustrated in the cutaway. It is interesting that grey matter is localized on the outer surface of the brain in a manner consistent with the hypothesis that NIR is beneficial to the grey matter. This area appears to warrant further research related to the effect of NIR on neuromelanin and blood flow within grey matter, especially in light of the daylighting studies that have shown that natural sunlight (NIR rich) improves the learning rates of children compared to artificial lighting (NIR deficient) (22).
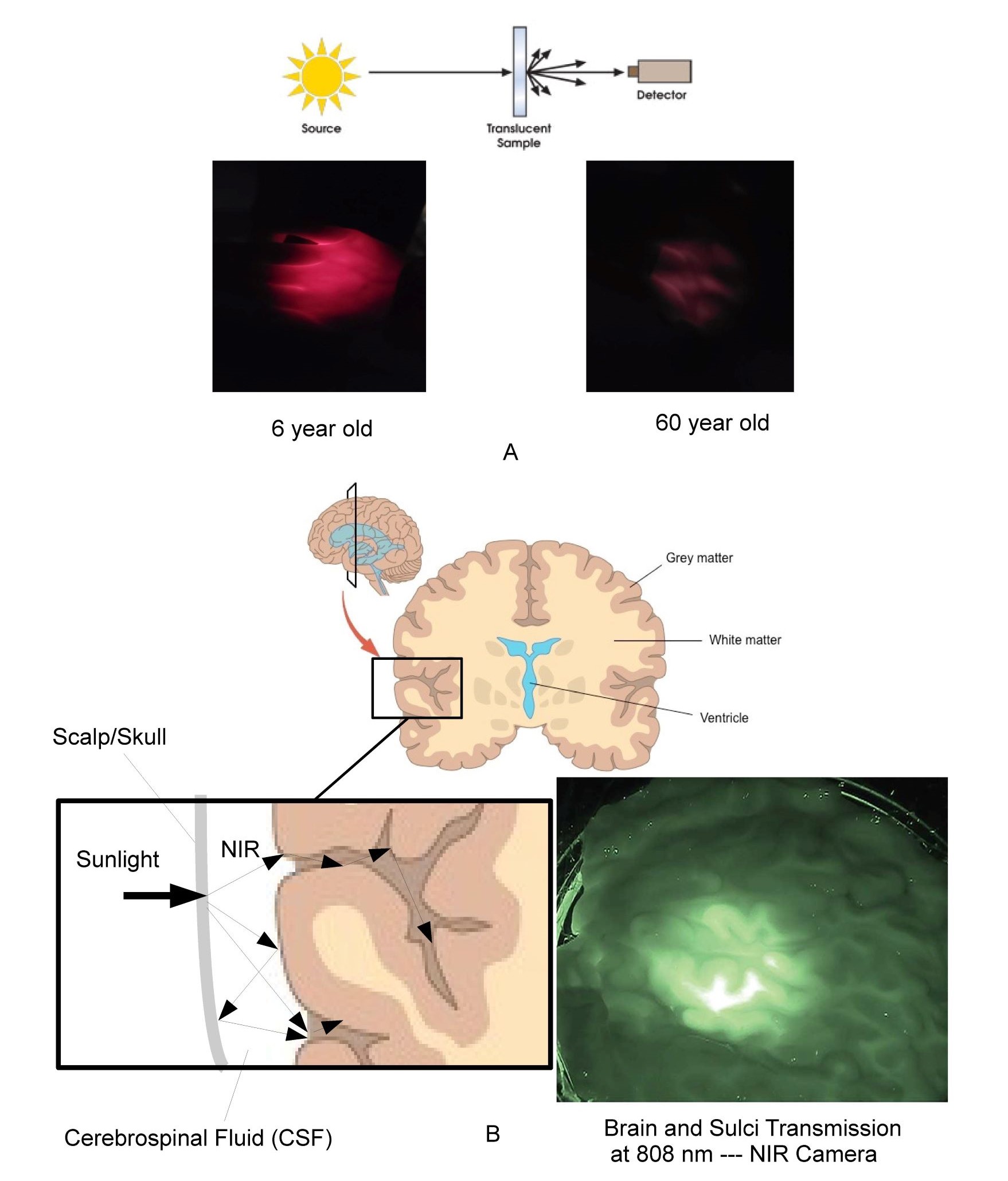
Fig. 5A typical source detector measurement technique and photos of relative visible transmission through the hand of 6 year-old compared to 60 year-old. Figure 5B illustrates the light-guiding and optical distribution effects of CSF along with NIR image of 1 inch (25 mm) thick slice of fresh human brain tissue illuminated by NIR sources.
The measurement of translucent materials is easily misleading due to scatter effects. It is however easy to show the relatively higher transmission of the body of a child compared to an adult. The brain, eye, and womb have addition complexity based on the formation of optical light-guiding features which efficiently funnel NIR photons in a manner similar to light-pipes used in most automotive and avionics instrumentation even into the furrows or sulci of the brain.
In general, optical modeling and imaging are required to correctly assess the number of cells impacted by various wavelengths in non-homogeneous translucent biological materials. Based on optical models the author has calculated that a significant number of NIR photons penetrate a minimum of 8 cm on average in the skin. This rough approximation was then used to calculate the number of cells impacted by NIR in adults, children, fetus and elderly as shown previously in Figure 4. A similar calculation was used for UV and Visible spectrums. These are approximations but clearly indicate that NIR is uniquely interacting with the majority of our cells throughout life. Also shown in the figure is a NIR image of a 25 mm thick cross-section of the human brain backlit with an 808 nm NIR source by Anders (23). The image shows transmittance through the brain tissue and along the sulci as expected by the models. The reader is encouraged to observe this effect personally by putting their thumb over the flashlight on their cellphone in a darkened room. The blue and green photons are strongly absorbed while the red photons emitted by the LED point source are uniformly distributed in the volume of the thumb. It should be noted that this conversion from a localized source to substantially uniform illumination within a large volume occurs within any scattering media that exhibits low optical absorption and anisotropic scatter coefficients. It is proposed that this effect occurs throughout the human body to provide substantially uniform stimulation to our blood vessels, grey matter, retina, and mitochondria. Due to their smaller physical size but similar optical absorption and scatter coefficients the models show that children and the unborn have higher exposure levels to the brain and internal organs than adults. This optical effect needs to be taken into consideration in any cognitive or learning situation. It is interesting that in melanin rich babies, pigmentation levels can take up to 6 months to reach their adult values after birth. One possibility being that the importance of NIR in the womb has been underestimated and that melanin levels are suppressed in the womb to maximize NIR exposure until birth. Based on this approximation, as we age, approximately 20 trillion of the 37 trillion cells that make up our body are exposed to NIR photons in natural sunlight (24). It is again noted that VOL (LED, OLED, and CFL) emits zero NIR.
From an optical perspective, the human body has developed some amazing mechanisms that appear to actively collect NIR photons and direct them to our most sensitive areas. As shown in Figure 6, the eye has a larger NIR transmission window than it does in the visible region.
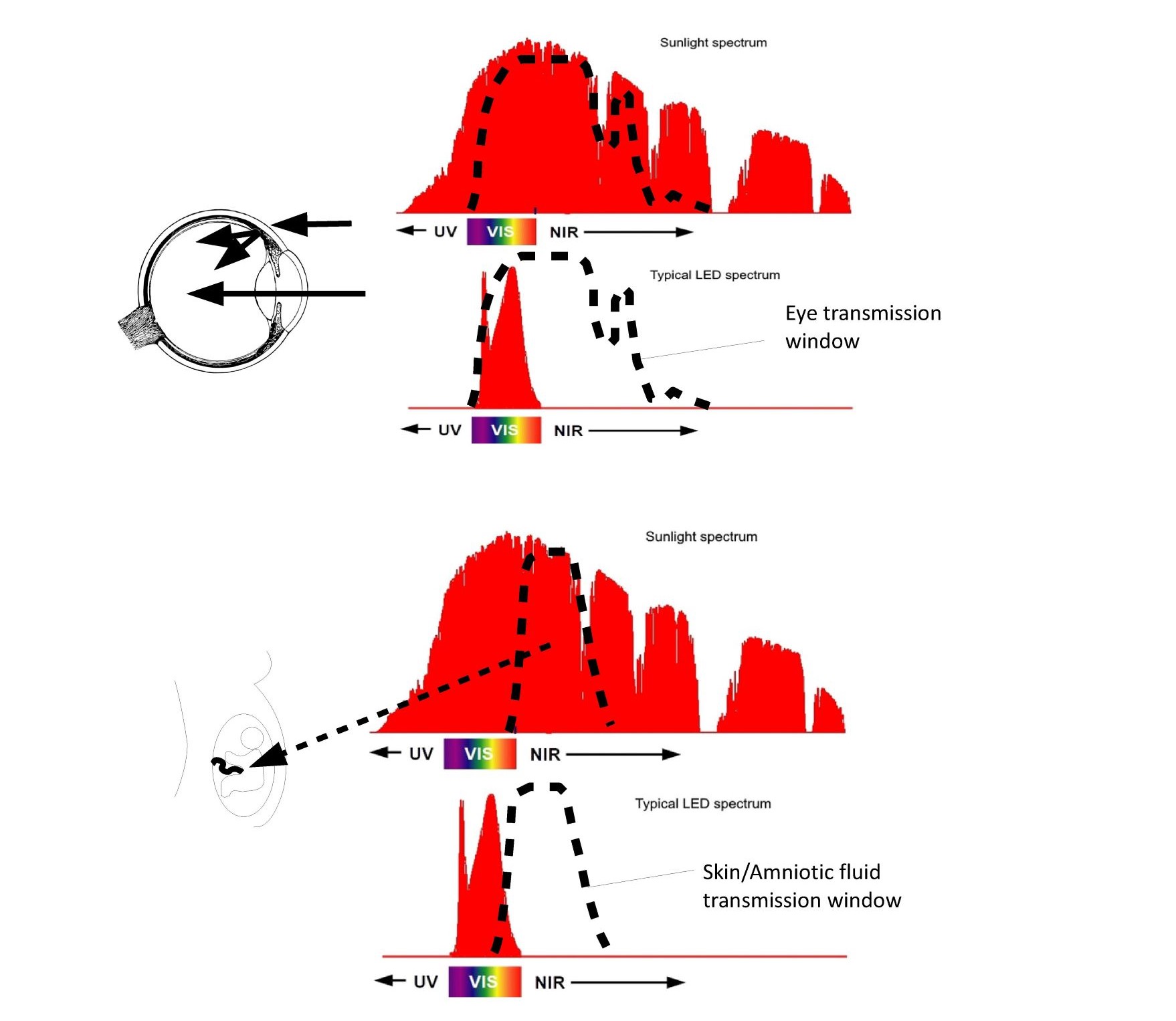
Fig. 6 NIR transmission windows of the eye and womb.
The eye optically is an imaging element in the VIS and a non-imaging element in the NIR with majority of the photons impinging on the retina entering through the eyelid and sclera. Similarly, the fetus is surrounded by the amniotic fluid which has a peak transmission in the NIR forming a dielectrically filled integrating sphere surrounding the fetus ensuring uniform exposure to NIR photons impinging on the mother’s belly.
The eyelid, sclera, and choroid (depending on melanin levels) transmit NIR such that the majority of the photons impinging on the retina actually don’t enter through the pupil but pass through the sclera flooding the retina with NIR photons even with our eyelids closed. Even more impressive is the optics of the fetus. Amniotic fluid has a peak transmission around 850nm similar to the CSF fluid around the brain that was previously discussed. The light-guiding of the amniotic fluid combined with the transmission of the mother’s skin has until recently bathed the fetus in NIR photons early in the pregnancy. As the pregnancy progresses the skin stretches allowing shorter wavelength photons to be exposed to the fetus later in the pregnancy. NIR photons have been shown to reduce MCP-1 proteins (25), while in a separate study, high MCP-1 proteins (associated with inflammation) in amniotic fluid have been linked to autism (26). As discussed previously CSF around the brain also has peak transmission in the NIR with multiple researchers pursuing the use of NIR treatments to treat Post Traumatic Stress Disorder (PTSD), dementia, and Parkinson (27). Once the optical ray tracing models are developed it is possible to estimate the three-dimensional free radical distribution using Electron Spin Resonance data provided by Zastrow (27). Zastrow measured the number of free radicals generated in skin as a function of wavelength as shown in Figure S2.
It is evident that UV photons are more effective at generating free radicals but all wavelengths generate significant amounts of free radicals. This is why the source spectrum and three-dimensional optical distributions discussed earlier are so critical to the problem. While UV photons may be more likely to create a free radical there are more blue photons in most light sources including the Sun, but especially LEDs. Conversely, blue photons penetrate deeper into the skin so they generate a lower density of free radicals than UV photons. In natural sunlight, UV photons (less than 430nm) and visible photons (greater than 430nm) generate equal amounts of free radicals as first realized by Zastrow et al (28). However, they did not combine their ESR data with the optical data to generate the three-dimensional free radical distribution, missing a fundamental difference between the type of free radicals being generated. It is proposed that the three-dimensional free radical density and location determines the degree of damage photons generate in biological systems.
Combining the optical absorption with the ESR action spectrum yields Figure 7, which is a representation of the three-dimensional free radical distribution in the skin which subcellular melatonin and other antioxidants battle every second of the day. It is evident that unlike UV photons, High Energy Visible (HEV) (400 -500nm) photons form free radicals in the fat rich dermis and sub-fat layers of the skin and retina. UV photons generate mainly reactive oxygen and nitrogen species in the outer layers of the skin which are replaced every 20 to 24 days. HEV photons generate large amounts of lipid free radicals in cells that have life cycles of months or longer. This represents a first step in understanding how photons interact with our cells. More detailed research is required to determine which free radicals are being generated, their location, and the body’s response to each type of free radical generated.
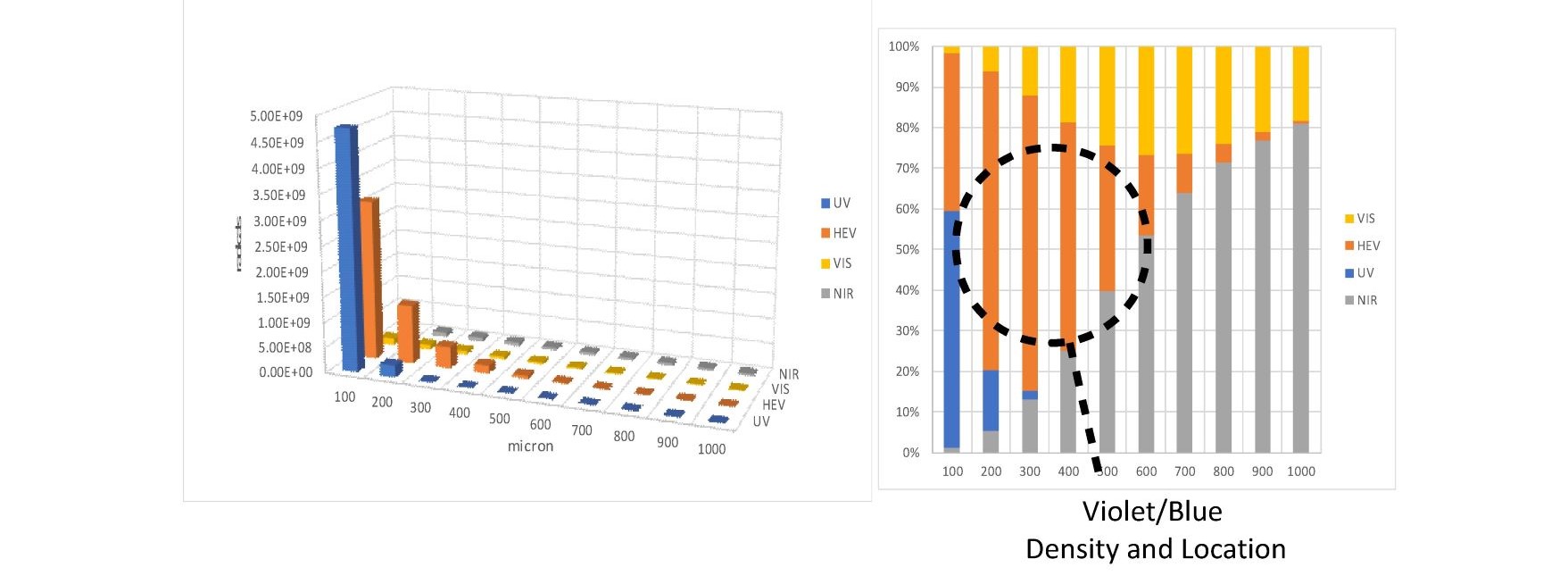
Fig. 7 comparison of free radicals generated at various skin depths by UV, HEV, VIS, and NIR photons.
An estimation of the number of free radicals generated and their location is presented. Due to the optical properties of melanin deficient skin HEV photons localize in the fat rich layers of the dermis and sub-fat layers leading to increased numbers of lipid free radicals.
This is an example of the insight that a three-dimensional mechanistic model can provide. Consider the impact of this graph on sunscreen manufacturers. For over 3 decades, skin cancer rates have continued to increase among Caucasians despite the development and widespread usage of UV blocking sunscreen (29). What the model indicates is that not only are equal amounts of free radicals generated by the UV and HEV/Visible photons as shown by Zastrow, but that the free radicals generated by the HEV photons are generating large amounts of lipid free radicals. The resulting lipid free radicals have half-lives of several seconds compared to most free radicals having half-lives of nanoseconds. As shown in Figure S3, again by Zastrow (30), the ratio of Lipid Oxygen Species (LOS) to Reactive Oxygen Species (ROS) increases based on dose as expected (accumulation). Sunburn is a natural body response that warns us to get out of the Sun, by blocking the UV but transmitting the HEV photons the public is misled into spending more time exposed to HEV photons. Lipid free radicals have been linked to skin cancer and other diseases (31). Under natural sunlight, the NIR photons have been shown to provide protection from the damage induced by both UV and visible photons in vivo and will be discussed later in the paper.
As a final point from the optical perspective, there is a general misconception that existing artificial lighting is benign with flux levels too low to generate damage. Figure S4 based on data from Zastrow (32) compares the number of free radicals generated by 750 lux of an office to up to 76,000 lux like a day on the beach in 30 minutes. From a dose standpoint, 8 hours in the office generates the same number of free radicals as 30 minutes laying out in the sun in accordance with the theory of reciprocity used in photochemistry. Based on personal inadvertent experience it is possible to get a “Blueburn” from handling LED lighting for extended periods of time. The result being approximately 1mm thick layer of skin dying and peeling off in good agreement with the model. In general, free radical generation rate of visible photons have been vastly under appreciated. What the ESR data and MBMs illustrates is that visible photons are not benign and may be a primary risk factor in certain skin cancers.
The majority of the literature from the lighting community is based on inexact two-dimensional units of measure such as lux, nits, etc., making it practically impossible to determine the applied dose. Zastrow does not provide the spectrum but does indicate the type of source used such that dose can be calculated approximately.
In general, all the bio-optical processes in the body are photochemical in nature and, as such, dose is what matters. There is a large body of research which uses NIR photons with measured dosage for treatments using terms such as Photobiomodulation (PBM), Low Level Laser Treatments (LLLT), and NIR phototherapy. In this field, dose matters and the narrowband emitters used allow for accurate comparisons between papers. The focus, however, is on developing treatments based on short term NIR exposures (20 minutes or less). Hamblin et al (33) has shown the ATP production is stimulated by non-thermal NIR exposures lasting a few minutes. It should be noted that a common misconception is that infrared equates to heat. Given that energy is inversely proportional to wavelength, thermally one mW/cm2 of 450nm (blue) photons and one mW/cm2 of 900 nm (NIR) photons generate the same temperature rise if the optical absorption coefficients are the same at both wavelengths. However, from a photochemistry standpoint one mW/cm2 of NIR photons exposes the body to twice as many NIR photons compared to one mW/cm2 of blue photons. Given that the 2nd law of photochemistry states that there is a one photon to one molecular bond relationship further illustrates the need to move to more exact units of measure that align with the underlying biological processes at play.
Keszler et al (34) has shown that NIR photons stimulate the dilation of blood vessels based on absorption in the blood vessel walls leading to the release of nitric oxide related compounds. Wang et al. (35) has used in vivo Broadband Near Infrared Spectroscopy to measure the impact of NIR Laser treatments on cytochrome c and oxygen levels in the blood with the intent to use NIR to treat Post Traumatic Stress Disorder (PTSD). In general, the complete solar spectrum provided by sunlight for billions of years has both positive and negative impact on cellular health. The return of rickets in some children based on blocking UV photons due to concerns of skin cancer is a recent example of how exposure to and lack of exposure to sunlight has both good and bad aspects. Given the variety and number of known chromophores and biological processes which respond to different wavelength ranges of sunlight, care must be taken before we alter the spectrum and intensity profiles from the sunlight that our cells assume we are exposed to.
Other optical studies that pertain to this field relate to how Visible Only emitters are negatively impacting health. Preliminary results for the A.B.C.D. study (36) indicate that children spending more than 7 hours exposed to artificial visible only light sources exhibited thinning of their cerebral cortex. Daylighting studies (37) show increased learning rates under natural sunlight compared to artificial lighting.
3. MELATONIN + OPTICS OF THE HUMAN BODY
Melatonin research from the lighting community has been almost exclusively focused on blue light (38). However, there are a few studies that recognize the impact of longer wavelength ranges on circulatory melatonin.
Figure 8 is from a study of athletes who were exposed for 20 minutes every evening for 14 days to 670nm over their entire body (16).
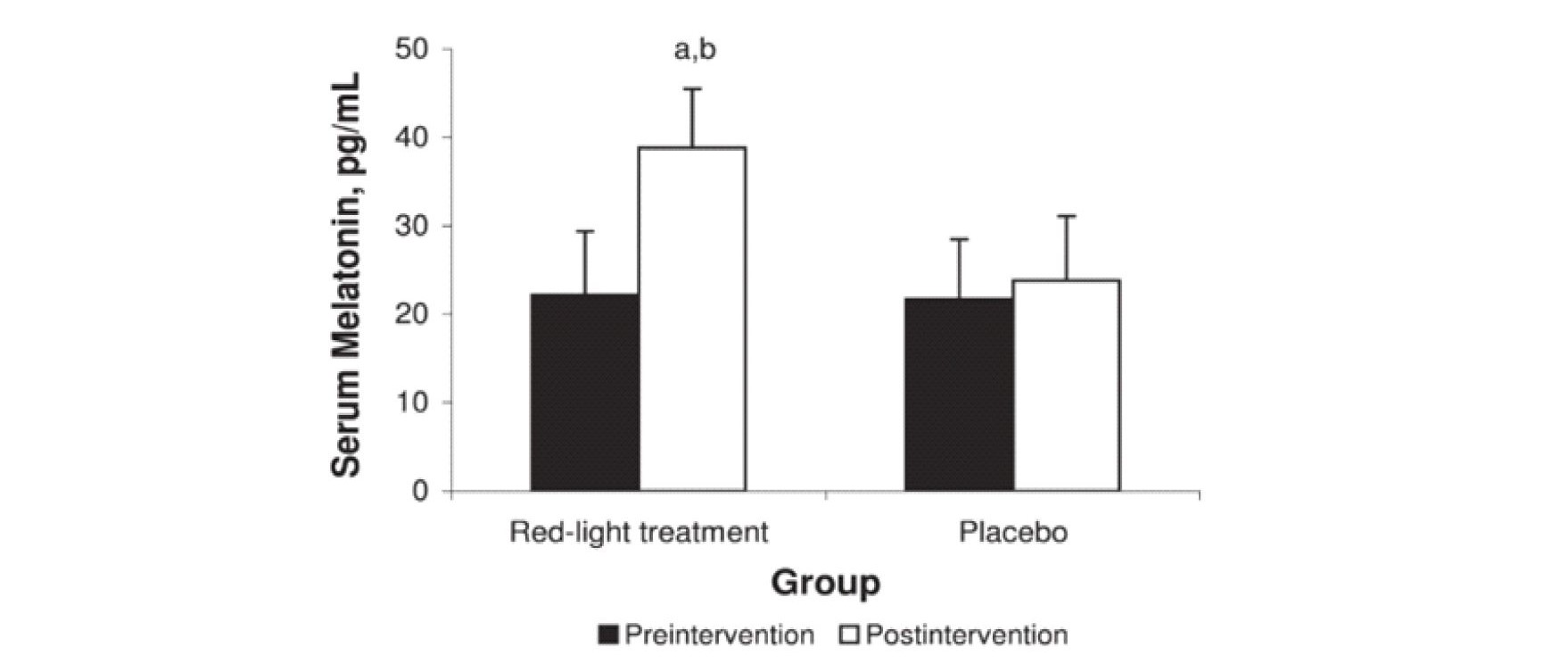
Fig. 8 Impact of red light on peak circulatory melatonin levels.
670 nm full body eyes covered exposure for 20 minutes each night for 14 days increased peak circulatory melatonin levels compare to control.
670nm (deep red) was used based on availability of treatment equipment. Unlike other studies the whole body was exposed and eyes were covered during treatment. Compared to the control, peak circulatory/serum melatonin levels were elevated as shown at the end of the test. In this study not only were the circulatory/serum melatonin levels increased but the test subject’s sleep quality and athletic performance levels also improved significantly.
This data is consistent with the hypothesis of this review in that the treatments stimulated high antioxidant levels in a large percentage of the athlete’s cells right before sleep (similar to sitting around a campfire) thereby reducing the amount of circulatory melatonin that was needed to be extracted from the blood during the night. In an earlier study, Figuerio and Rae (39) showed similar effects at 630nm but failed to recognize the significance of the results. It should be noted that the biological or therapeutic window in the NIR extends from approximately 650nm to 1200 nm such that small changes in wavelength in the red region can significantly impact the optical absorption profile in the body. Like Zastrow, Figuerio and Rae failed to take the effect of the three-dimensional optical distribution into account. In both these cases the increase in the peak circulatory melatonin levels support the hypothesis of this paper. Additionally, it has been shown that 808nm NIR exposure along with melatonin treatments synergistically improve healing rates in bones (40). The results of Figure 8 are based on exposure of the full body with covered eyes for 20 minutes each evening which maximized the total number of cells exposed and appears to positively impact circulatory melatonin. It however should be noted that most light clothing and dyes exhibit high transmission in the NIR allowing for significant exposure even when wearing clothing.
Much of the literature associated with melatonin has focused on supplements (41) taken orally or applied via skin creams. Papers on extrapineal or subcellular melatonin are however becoming more prevalent in the literature associated with subcellular melatonin’s impact on aging (42). The stimulation of subcellular melatonin using NIR treatments has even been proposed as a mechanism to extend lifespans (43). In general, subcellular melatonin is a primary candidate for local control and response to free radicals either generated during normal energy production or due to external free radical generating sources like sunlight. The ability to quantify the number, location, and type of free radicals being generated is required to understand how subcellular melatonin is used in the body.
As shown in Figure 9, it is necessary to understand the optics of the human body to quantify the free radical impact and antioxidant response. In nature, our most sensitive organs were daily exposed to predominately NIR photons throughout the day. Melatonin is one the body’s most effective antioxidants. Melatonin provides the possibility of two unique biomarkers in that it is now known to have both endocrine and autocrine actions. The exclusive use of circulatory melatonin as a biomarker has to be used with caution and appears to have led the lighting and display industry to develop and produce light sources that increase oxidative stress in the retina, skin, brain, etc. The need exists for accurate biomarkers of cellular antioxidant health. Such studies should focus on developing products that optimize subcellular melatonin. As shown, NIR uniquely interacts with the majority of our cells. Even the brain’s surrounding fluids have peak transmission of the NIR optically forming a light-guiding effect. This coupled with the higher NIR absorption of neuromelanin in the gray matter compared to the underlying white matter of the brain creates an interesting collection mechanism from an optics perspective.
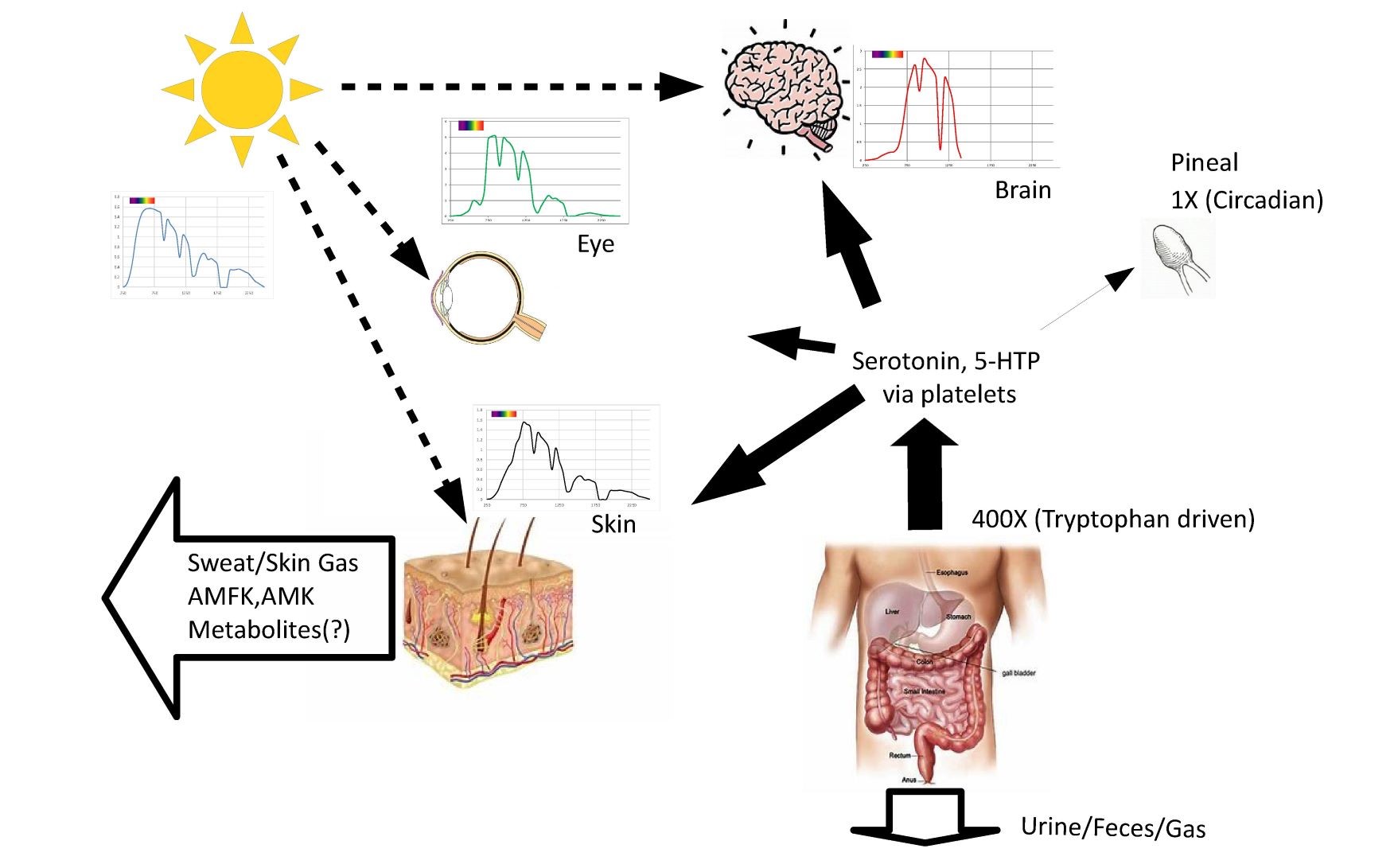
Fig. 9 illustration of example spectrums for the skin, retina, and brain along with proposed relationship to melatonin production and waste products.
Sunlight for millions of years was the largest external stimulus to the human body. Subcellular melatonin has been shown to be produced in orders of magnitude higher quantities than the pineal gland produces. It is proposed that the body generates an antioxidant reservoir stimulated in part by sunlight exposure during the day.
Figure S5 illustrates the speculation that during the day subcellular melatonin is produced in excess, essentially acting as a reservoir or battery storing up an excess of antioxidant capacity during the night, circulatory melatonin from the pineal gland allows for efficient delivery of supplemental melatonin to only those cells most in need.
As stated earlier, NIR photons has been shown to stimulate dilation and increase blood flow in the skin, retina, brain, fetus, etc. As illustrated above, because NIR photons uniquely penetrates deep into the body, the majority of the capillary network extending centimeters into the body are stimulated to release nitric oxide related compounds increasing blood flow and waste removal rates. This is an example of how the body is designed based on the assumption that we are exposed to a predominately near infrared emitter. The body assumes that UV and visible photons are localized in our outer skin layers generating damage based on the density of free radicals where the free radical density is determined by the optical properties of the underlying cells. Under natural sunlight, it is apparent that NIR provides a proportional and local response that increases waste removal rates and stimulates antioxidant response. As discussed by Slominski et al (44), melatonin also provides immunomodulatory, thermoregulatory, and antitumor properties. NIR and subcellular melatonin appear to work together.
Figure S6 illustrates further why the optical properties must be taken into account to understand biological processes. The blood vessels are observable in the NIR because of the low optical NIR absorption of skin compared to the high optical NIR of the hemoglobin in the blood. The associated scattering and low absorption of the surrounding tissues localizes the NIR photons in the blood vessels as shown in the pictures. In the visible wavelength range the blood vessels cannot be seen. In the NIR wavelength range the blood vessels are readily apparent. Given that NIR photons penetrate several centimeters into the body there are other optical mechanisms we haven’t even thought of yet to be discovered.
Figure S7 illustrates that the human body is constantly being exposed to a changing environment. It has been shown that NIR photons provide up to a SPF 15 level of skin protection (45) if skin is pretreated prior to exposure to UV photons. In general, this work illustrates the need for increased rigor and collaboration with medical researchers by the lighting community. It is possible to three-dimensionally quantify the effect of photons on biological systems. As illustrated previously, there are huge differences in the optical absorption and scatter coefficients as a function of wavelength in the human body. Simply changing the wavelength from 630nm to 670nm radically increases the number of cells impacted. As long as we continue to use inexact units of measure and two-dimensional empirical approaches we will not get to the solution. Much of the confusion in the lighting field is simply based on not understanding the optics of the human body.
This broader view of circadian theory takes into account both circulatory and subcellular melatonin. Circulatory melatonin is stimulated by the absence of light. But subcellular melatonin may be stimulated by the presence of light. Again, the optics have to be taken into account. In the morning, the sunrise is predominately NIR photons which in this hypothesis and based on Zhao et al (16) stimulates high levels of antioxidants in our cells. As noon approaches the number of UV and visible photons we are exposed to increases. Roughly NIR/VIS optical watt ratios are approximately 3 to 1 at sunrise, reaching 1 to 1 at noon, returning to 3 to 1 at sunset. Campfires and incandescent light sources have NIR/VIS ratios of 10 to 1. So as the day wanes, the relative number of NIR photons again increases and for 600,000 years people gathered around campfires
(tons of NIR) before bedtime. More recently, reading a book under candle light or using an incandescent bulb guaranteed large amounts of NIR exposure before sleep. That no longer occurs in modern society. Instead, the normal day involves getting up under Visible Only emitters, spending the day in an office with Visible Only emitters and windows coated to block NIR photons from entering while looking at high information content Visible Only displays, and return home after dark to a home filled with Visible Only Emitters (lighting and displays) which suppress circulatory melatonin and inhibit a good night’s sleep. Over the course of years, it is reasonable to argue that this leads to molecular debris accumulation and a host of diseases.
It should be noted that all the discussion to this point has ignored the impact of skin pigmentation. Less than a 2X change in yearly sunlight exposure compelled the body to generate a greater than 10X difference in melanin levels (1.3% to 43%) presumably because sunlight was so important to survival. Lighting is designed for melanin deficient individuals. Melanin rich individuals are adversely impacted by that choice. Melanin rich individuals at higher latitudes do not receive sufficient UV or NIR exposure compared to lower latitudes. Figure 10 illustrates the optical difference (non-sequential optical ray trace) between melanin rich (43%) and melanin deficient (1.3%) individuals. It is presently unknown how the free radical generation rates vary with skin color. Kim et al (46) has measured differing levels of melatonin and its metabolites in skin cells in different ethnic groups under UV only exposure with the highest melatonin levels being in young African Americans and older Caucasians. As previously discussed, ESR data shows that in natural sunlight the UV and visible portions generate equal amounts of free radicals but at different densities due to optical penetration depth. With sufficient combined research it should be possible to quantify the differences and to generate optimized lighting solutions for everyone.
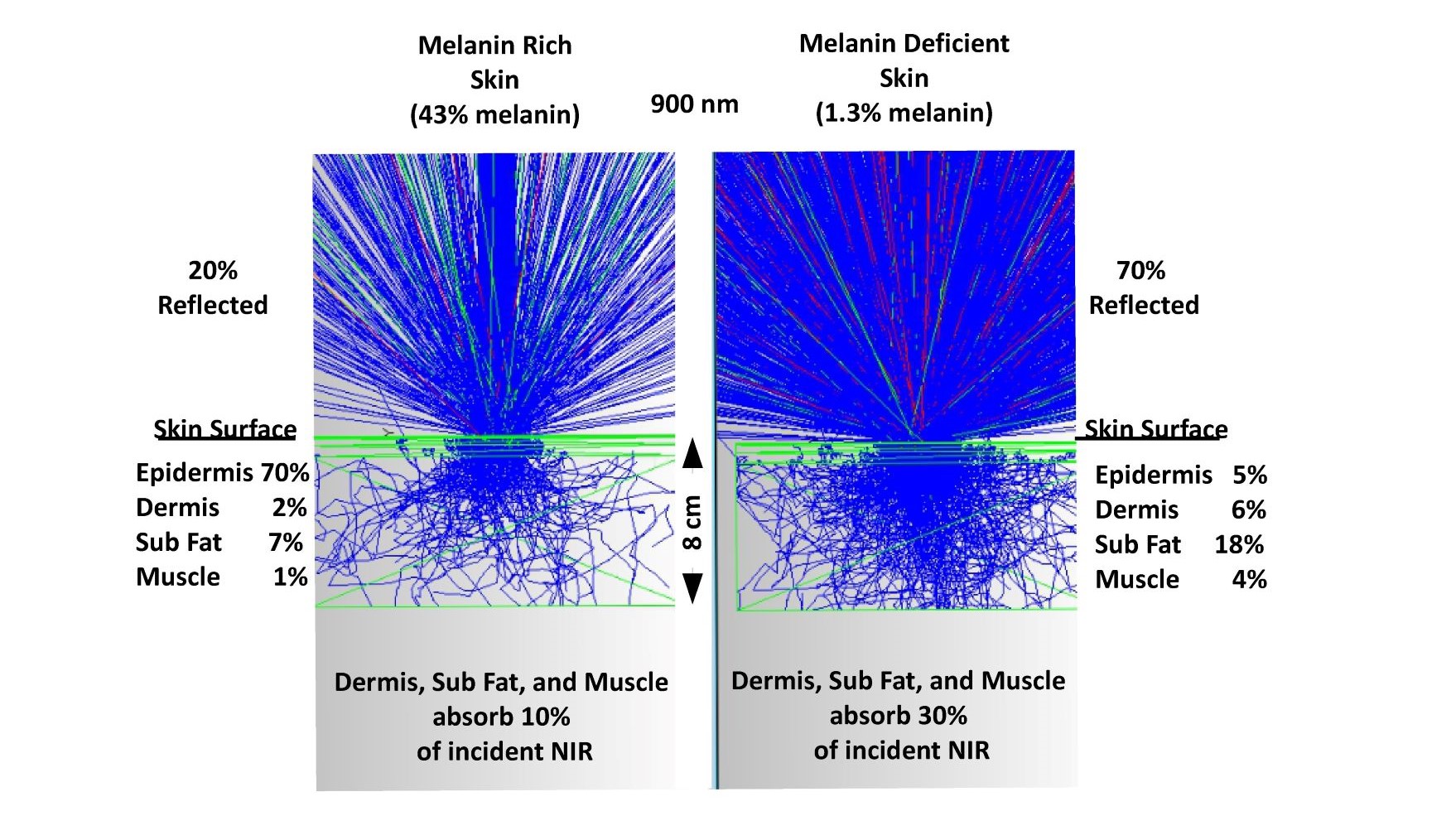
Fig. 10 a comparison of melanin rich and melanin deficient optical skin models in the NIR
To understand how photons propagate in the human body it is necessary to take into account a wide range of optical parameters. As shown, melanin rich individual have much different absorption profiles in their skin but still receive significant levels of exposure even 8 cm into the body.
Also, not discussed is the important topic of pulsed versus fixed emitters. As the same time the spectral range is shrinking, the information content is radically increasing exposing especially children to high frequency pulsed light. Fundamentally, we receive this high information content from display devices in the form of pulsed streams of photons. Significant research opportunities are possible regarding the frequency response of biological systems. As shown by Barolet and Boucher (47), pulsed emission increases biological activity depending on the process excited. The electron transport chain (ETC) and production of ATP is a bio-mechanical process with rotational rates of over 600 rotations per second. Cochlear excitation via laser diodes have been also shown to involve microsecond response times. In general, more research is required to understand the frequency response of biological systems as we pursue higher information content. Given the recent A.B.C.D. study that indicates that thinning of the cerebral cortex in children spending more than 7 hours exposed to displays, it would appear worthwhile to study how high frequency content emitters are impacting cell physiology. Both the direct impact and the substitutional effects must be taken into account. The basic stimuli causing these changes to the structure of the brain are pulsed photons. It is proposed that pulse width modulated lighting in particular is an unnecessary oxidative stress source and that the re-introduction of NIR photons to our artificial environment may provide a significant level of protection, especially for children, against the negative impact of higher information content.
4. CONCLUSION
Melatonin and sunlight are intimately linked. This love/hate relationship is further compounded by the existence of at least two forms of melatonin, circulatory and subcellular. It is proposed that one is produced in response to the absence of sunlight while the other is produced in response to the presence of sunlight. Circulatory melatonin and its source, the pineal gland, operate systemically (endocrine) based on a narrowband stimulus in the violet, blue, and green region of the visible spectrum. It has been proposed that subcellular melatonin is produced in much larger amounts in most of the cells of the body and its functions are autocrine/paracrine in nature (48). To further understand this area of research it is important that disciplines work together.
Figure 11 contrasts the work of Hill et al (49) (Biology) and Zhang et al (50) (Optics). Hill and colleagues show that very low levels of blue light can lead to higher breast cancer tumor growth rates by suppressing melatonin, while Zhang is looking at the optical absorption of breast cancer cells finding that they contained more water than healthy breast cells and therefore absorb strongly in the NIR and IR spectrum possibly allowing for selective heating of cancer cells. A biologist and an optics person are working at opposite ends of the solar spectrum fighting the same disease.
Imagine what they could do working together.
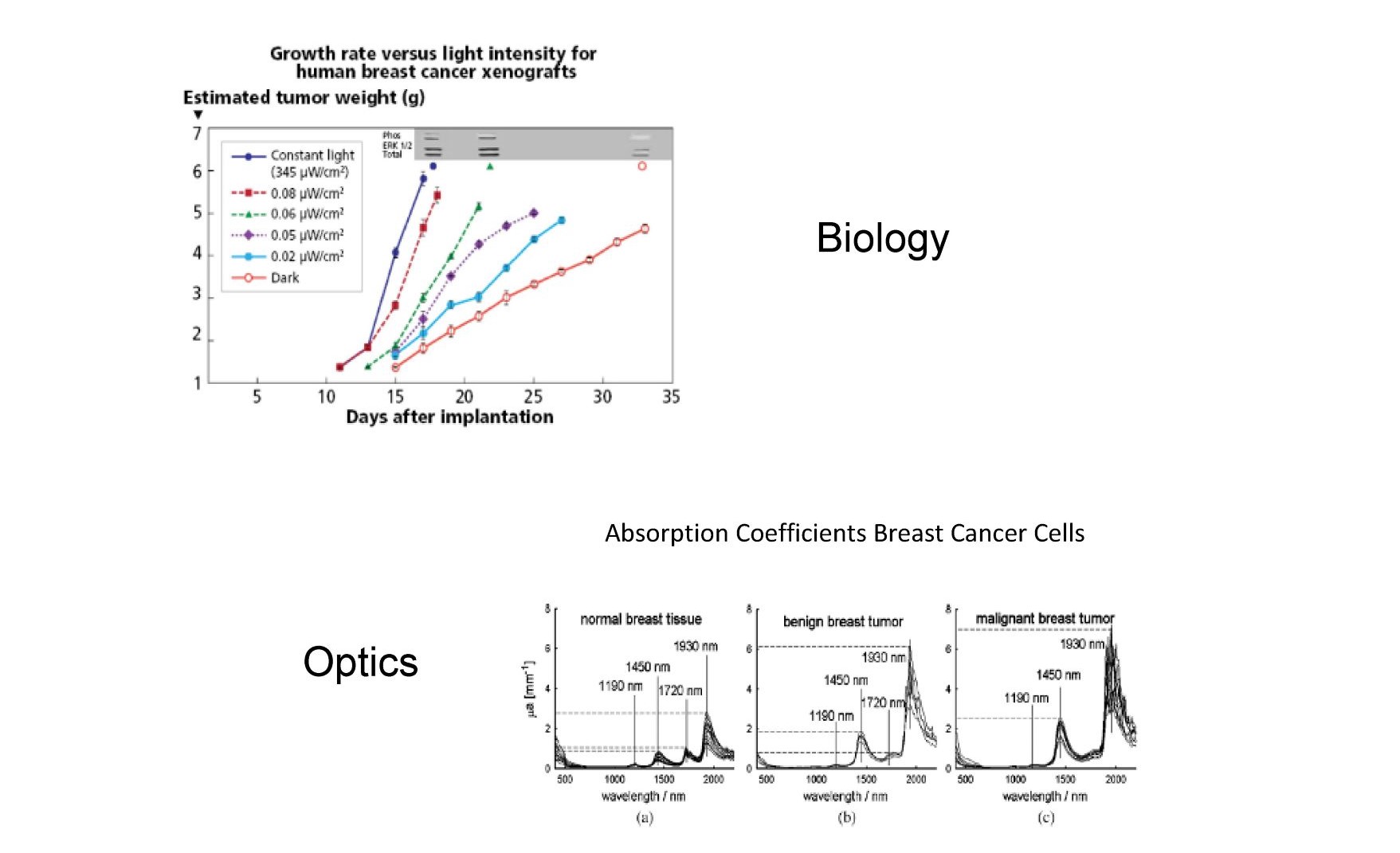
Fig. 11 the impact of blue photons on cancer cells and the increased optical absorption of infrared photons in the same cancer cells.
Breast cancer cell growth rates increase dramatically when exposed to HEV photons (blue light) while on the other end of the solar spectrum breast cancer cells strongly absorb infrared photons compared to healthy breast cells due to lower lipid levels in cancer cells. Under natural sunlight both occur, but in our modern society only the first occurs due to the elimination of NIR/IR from our artificial environment. Does infrared suppress cancer cells?
Sunlight and the optical properties of our surroundings guaranteed that for millions of years most cells in the human body, especially in children, were always exposed to predominately NIR photons during the day, and for 600,000 years as groups gathered around the campfire in the evening before bedtime. This continued for the last 150 years based on the excessive amounts of NIR emitted by incandescent bulbs. Fifty years ago, fluorescent bulbs started to eliminate NIR from the artificial environment, but the incandescent lights continued to provide NIR, especially in homes. Recent government mandates have begun a process that will, for the first time in the history of our species, eliminate most of the NIR exposure that once dominated, as shown in Figure S8.
These changes are being driven by government mandates and will be universally implemented over the next couple of decades. With 90% of our time under artificial lighting and in front of displays that emit zero NIR, and with NIR blocking window treatments preventing NIR from entering our offices, schools, and homes, modern societies have created NIR caves. As Figure S9 illustrates, most researchers are aware that we are eliminating night by exposing ourselves to excessive amounts of visible emission in our signage, streetlights, headlights, lighting, computer screens, and displays. What most researchers do not realize, is that for the first time, 70% of the spectrum emitted by the Sun (NIR) is being eliminated from our lives during the day.
Melatonin may hold the key to better health if an accurate subcellular melatonin biomarker can be identified. Melatonin and sunlight are intimately linked. This relationship has been going on for several million years in hominids. Over the last century modern society has been slowly eliminating night from our lives. Most researchers agree that increased exposure to blue photons at night from modern light sources is suppressing circulatory melatonin leading to less sleep and indirectly to a host of diseases. By modern society eliminating NIR from offices, homes, and schools we are directly impacting cellular processes. It has been shown optically that NIR photons uniquely interact with a majority of our cells. Optically, the body appears to be designed to localize NIR photons in some of the most sensitive areas, including the retina, brain, and fetus. It has been proposed, based on a review of the biological and optical literature and the results from three-dimensional Mechanistic Bio-optical Models, that NIR stimulates each cell to generate an excess of antioxidants locally including melatonin biosynthesis in the skin (44). Melatonin is a powerful and ubiquitously acting antioxidant probably produced in all cells (1). Subcellular melatonin appears to uniquely operate as an autocrine/paracrine antioxidant, isolated by the cellular membrane from the endocrine antioxidant circulatory melatonin Given that melatonin has been shown to control other antioxidants and antioxidant enzymes, it may provide the key biomarker to determining how to create the best artificial environment.
As the Roundup™ lawsuits clearly illustrate, there is an added level of responsibility and liability associated with globally altering the publics’ environment. As shown above, the human body has evolved and adapted processes based on the assumption that it is exposed daily to a single, predominately Near Infrared broadband fixed emitter (Sun). That assumption is no longer valid. Over the next several decades, the government will convert all lighting to Visible Only emitters, with little understanding as to the long-term health consequences.
In nature, the human body is never exposed to UV or visible photons without an excess of NIR photons. It would appear there are a host of reasons why. This work suggests that melatonin is once again at the core of human health, and that the lighting, display, and architectural industry should be focused on developing products that enhance the amount of subcellular melatonin in as many of our cells as possible, e.g. the skin, retina, fetus, brain, etc. To do so, simply means that the public should be provided with more of what the Sun has been providing for billions of years. Ironically, this lesson has already been learned by the cannabis industry, where they are being forced to reintroduce NIR to produce healthy flowering plants. The human body deserves the best artificial environment possible, so that children can learn faster and we all can live healthier lives as we age.
ACKNOWLEDGEMENT
No external funding sources were used in this review.
AUTHORSHIP
S. Zimmerman contributed to the conception and drafting of the manuscript. R.J. Reiter contributed to discussion and editing of the manuscript.
CONFLICT OF INTEREST
Scott Zimmerman is CEO of Silas Inc. which is developing lighting solutions based at least in part on the models discussed in this review. Prof. Reiter has no conflict of interest.
REFERENCES
Tan DX, et al. (2013) Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res. 54: 127-138
Suofu Y, et al. (2017) Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. PNAS 114 (38): E7997-E8006.
He C, Wang J, Zhang Z, Yang M, Li Y, Tian X, Ma T, Tao J, Zhu K, Song Y, Ji P, Liu G (2016). Mitochondria Synthesize Melatonin to Ameliorate Its Function and Improve Mice Oocyte's Quality under in Vitro Conditions. Int. J. Mol. Sci. 17 (6): E939. doi: 10.3390/ijms17060939.
Wang L, Feng C, Zheng X, Guo Y, Zhou F, Shan D, Liu X, Kong J. (2017) Plant mitochondria synthesize melatonin and enhance the tolerance of plants to drought stress. J. Pineal Res. 63 (3): E12429. doi: 10.1111/jpi.
Odinokov D, Hamblin MR (2018) Aging of lymphoid organs: Can photobiomodulation reverse age-associated thymic involution via stimulation of extrapineal melatonin synthesis and bone marrow stem cells? J. Biophot. 11 (8): e201700282.
Dimakatso M et al (2018) Role of photobiomodulation on the activation of the smad pathway via TGF-βin wound healing. J. Photochem. Photobio. B: Bio. 189: 138-144
Hamblin M (2016) Shining light on the head: photobiomodulation for brain disorders. BBA Clinical 6: 113-124.
Merry GF, et al (2017) Photobiomodulation reduces drusen volume and improves visual acuity and contrast sensitivity in dry age-related macular degeneration Acta Ophthalmol. 95 (4): e270-e277
Cassano P, et al (2018) Transcranial photobiomodulation for the treatment of major depressive disorder, the elated-2 pilot trial. Photomed. Laser Surg. doi: 10.1089/pho.2018.4490.
Reiter RJ, et al. (2017) Melatonin as a mitochondria-targeted antioxidant: one of evolutions best ideas. Cell Mol. Life Sci. 74: 1863-1881
Bonnefort-Rousselot D, Collin F (2010) Melatonin: Action as antioxidant and potential applications in human disease and aging. Toxicology 278 (1): 55-67.
Slominski A, et al. (2018) Melatonin: A cutaneous perspective on its production, metabolism, and functions. J. Invest. Derm. 138 (3): 490-499.
Blasiak J, et al. (2016) Melatonin in retinal physiology and pathology: The case of age-related macular degeneration. Oxid. Med. Cell Longev. 2016: 6819736
Acuna-Castroviejo D, et al. (2014) Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol. Life Sci. 71 (16): 2997-3025. doi: 10.1007/s00018-014-1579-2.
Koltover V (2017) Free radical timer of aging: from chemistry of free radicals to systems theory of reliability. Curr. Aging Sci. 10 (1): 12-17.
Zhao J, et al. (2012) Red light and the sleep quality and endurance performance of chinese female basketball players. J. Athletic. Train. 47 (6): 673-678.
Kumar J, et al. (2015) Circadian rhythms in glucose and lipid metabolism in nocturnal and diurnal mammals. Mol. Cell Endocrinol. 418: Pt 1:74-88.
Kumar H, et al. (2012) the role of free radicals in the aging brain and the Parkinson’s disease: convergence and parallelism. Int. J. Mol. Sci. 13 (8): 10478-10504.
Bashkatov A, et al. (2006) Optical properties of human cranial bone in the spectral range from 800 to 2000 nm. Proc. of SPIE 6163: 616310-9
Borjigin J, et al. (2012) Circadian regulation of pineal gland rhythmicity. Mol. Cell Endocrinol. 349 (1): 13-19.
Tirpak A, Young R (2008) Accurate transmission measurements of translucent materials. Photonics Spectra https://www.photonics.com/Article.aspx?AID=32297.
Shishegar N, Boubekri M (2016) Natural light and productivity: analyzing the impacts of daylighting on students’ and workers’ health and alertness. Conference: Int. Conf. on “Health, Biological and Life Science” (HBLS-16), at Istanbul, Turkey.
Anders J. (2017) Photobiomodulation therapy comes of age. BioPhotonics 24 (2): 28
Bianconi E et al. (2013) An estimation of the number of cells in the human body. Ann. Hum. Biol. 40(5): 463-471.
Kuboyama N, Abiko Y (2012) Reduction of monocyte chemoattractant protein-1 expression in rheumatoid arthritis rat joints with light emitting diode. Laser Ther. 21 (3): 177-181.
Abdallah M, et al. (2012) Amniotic fluid chemokines and autism spectrum disorders: An exploratory study utilizing danish historic birth cohort. Brain Behav. Immun. 26 (1): 170-176.
Yennu A, et al. (2016) Prefrontal responses to stroop tasks in subjects with post-traumatic stress disorder assessed by functional near infrared spectroscopy. Sci. Rep. 6: 30157. doi: 10.1038/srep30157
Zastrow L, et al. (2009) Detection and identification of free radicals by UV and visible light in ex vivo human skin. Intl. J. Cos. Sci. 31 (5):207-215.
Guy G, et al. (2015) Vital Signs: Melanoma incidence and mortality trends and projections – united states 1982 to 2030. MMWR June 2015
Zastrow L, et al. (2015) Free radical threshold value: A new universal body constant. Skin Pharm. Phys.28 (5): 264-268.
Haywood R, et al. (2007) Protein, lipid, and DNA radicals to measure skin UVA damage and modulation by melanin. Free Rad. Bio. Med. 44 (6): 990-1000.
Zastrow L, et al. (2015) Free radical threshold value: A new universal body constant. Skin Pharm. Phys.28 (5): 264-268.
Hamblin M, et al. (2018) Low-level light therapy: Photobiomodulation. SPIE Press Book
Keszler A, et al. (2017) Red/near Infrared light stimulates release of an endothelium dependent vasodilator and rescues vascular dysfunction in diabetes model. Free Rad. Biol. Med. 113: 157-164. doi: 10.1016/j.freeradbiomed.2017.09.012.
Wang X, et al. (2016) Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser. Sci. Rep. 6: 30540.
Adolescent Brain Cognitive Development Study. https://abcdstudy.org
Yacan S. (2014) Impact of daylighting on preschool students’ social and cognitive skills. Thesis U of Nebraska 2014.
Lockley S, et al. (2003) High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J. Clin. Endocrinol. Metab. 88 (9): 4502-4505
Figueiro M, Rea M (2010) The effects of red and blue lights on circadian variations in cortisol, alpha amylase, and melatonin. Int. J. Endocrinol. 2010: 829351. doi: 10.1155/2010/829351.
Son J, et al. (2017) A novel combination treatment to stimulate bone healing and regenerate under hypoxic conditions: Photobiomodulation and melatonin. Lasers Med. Sci. 32 (3): 533541.
Abbaszadeh A, et al. (2017) Melatonin role in ameliorating radiation-induced skin damage: From theory to practice (a review of literature). J. Biomed. Phys. Eng. 7: 127-136.
Popovic B, et al. (2018) The influence of ageing on the extrapineal melatonin synthetic pathway. Exp. Gerontol. 110: 151-157.
Odinokov D, Hambiln MR (2018) Aging of lymphoid organs: Can photobiomodulation reverse age-associated thymic involution via stimulation of extrapineal melatonin synthesis and bone marrow stem cells? J. Biophot. 11 (8): e201700282.
Slominski AT, et al Melatonin, mitochondria, and the skin. Cell Mol. Life Sci. 74 (21): 39133925.
Barolet D, et al. (2016) Infrared and skin: friend or foe. J. Photochem. Photobio. B: Bio. 155 : 78-85
Kim TK, et al. (2015) Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro. Mol. Cell Endocrinol. 404: 1-6.
Barolet D, Boucher A (2008) LED photoprevention: reduced MED response following multiple LED exposures. Lasers Surg. Med. 40 (2): 106-112.
Skobowiat C, et al. (2018) Melatonin and its derivatives counteract the ultraviolet B radiation induced damage in human and porcine skin ex vivo. J. Pineal Res. 65: e12501. doi: 10.1111/jpi.12501.
Hill SM, et al. (2015) Melatonin: An inhibitor of breast cancer. Endocr. Relat. Cancer 22 (3): R183-R204.
Zhang Y, et al. (2013) Visible and near-Infrared spectroscopy for distinguishing malignant tumor tissue from benign tumor and normal breast tissues in vitro. J. Biomed. Optics 18 (7): 077003.
This work is licensed under Creative Commons Attribution 4.0 International License