Please cite this paper as:
Shukla, M., Sotthibundhu, A. and Govitrapong, P. 2020. Role of melatonin in regulating neurogenesis: Implications for the neurodegenerative pathology and analogous therapeutics for Alzheimer’s disease. Melatonin Research. 3, 2 (Jun. 2020), 216-242. DOI:https://doi.org/https://doi.org/10.32794/mr11250059.
Review
Role of melatonin in regulating neurogenesis: Implications for the neurodegenerative pathology and analogous therapeutics for Alzheimer’s disease
Mayuri Shukla1, Areechun Sotthibundhu2, Piyarat Govitrapong1*
1Chulabhorn Graduate Institute, Chulabhorn Royal Academy, Bangkok 10210, Thailand
2Chulabhorn International College of Medicine, Thammasat University, Patumthani, 12120, Thailand
*Correspondance: piyarat.gov@mahidol.ac.th, piyarat@cgi.ac.th, Tel: +6625541900 ext. 2415
Running title: Melatonin in regulating neurogenesis
Received: January 31, 2020; Accepted: May 30, 2020
ABSTRACT
The revelation of adult brain exhibiting neurogenesis has established that the brain possesses great plasticity and that neurons could be spawned in the neurogenic zones where hippocampal adult neurogenesis attributes to learning and memory processes. With strong implications in brain functional homeostasis, aging and cognition, various aspects of adult neurogenesis reveal exuberant mechanistic associations thereby further aiding in facilitating the therapeutic approaches regarding the development of neurodegenerative processes in Alzheimer’s Disease (AD). Impaired neurogenesis has been significantly evident in AD with compromised hippocampal function and cognitive deficits. Melatonin the pineal indolamine augments neurogenesis and has been linked to AD development as its levels are compromised with disease progression. Here, in this review, we discuss and appraise the mechanisms via which melatonin regulates neurogenesis in pathophysiological conditions which would unravel the molecular basis in such conditions and its role in endogenous brain repair. Also, its components as key regulators of neural stem and progenitor cell proliferation and differentiation in the embryonic and adult brain would aid in accentuating the therapeutic implications of this indoleamine in line of prevention and treatment of AD.
Key words: melatonin, neurogenesis, neurodegeneration, Alzheimer’s disease.
__________________________________________________________________________
1. INTRODUCTION
Neurodegenerative diseases are characterized by functional disintegration of newly generated neurons which are associated with cognitive and memory dysfunctions. The theory of Alzheimer’s disease (AD) pathogenesis as an event of deregulated cellular differentiation was first proposed by Santiago Ramon and Cajal, S. and has been discussed in terms of degeneration and regeneration of the nervous system (1). The dramatic decrease in neurogenesis during adulthood and its further decline with advance aging (2) suggest that reduced proliferation of neural progenitor cells might be one of the important mechanisms associated with the cognitive decline observed in AD subjects and the fact that cell cycle kinetics and the transition from proliferation to cell cycle exit and differentiation governs major aspects of neurogenesis (3) with recent evidence of drastic decrement in adult hippocampal neurogenesis in AD patients (4), strongly recommends this area to be a key target for developing anti-AD therapeutics (5).
Melatonin, the multifunctional indolamine acts as an endogenous factor that modulates and promotes plasticity in the brain and has been identified in the regulation of hippocampal neuronal development during adulthood. Along with promoting endogenous neurogenesis (6, 7), melatonin also regulates hippocampal plasticity (8, 9) both at structural and functional levels (10). Studies have affirmed that exogenous melatonin acts during different events of the neurogenic process which involves the activation of its membrane receptors distributed abundantly in the hippocampal neurogenic niches (11). Additionally, cumulative evidence has pointed out that melatonin receptors not only play important physiological roles in sleep, anxiety, pain and circadian rhythm, but might also be involved in the pathogenesis of several neurodegenerative diseases including AD (12). The significance of melatonin in brain can be marked by a study which demonstrated that how the absence of maternal pineal melatonin might determine abnormal brain programming in the offspring with implications for brain function and behavior (13). Nonetheless, melatonin exerts neuroprotective effects in the central nervous system (CNS) and its age-related decline itself explains its regulatory effects on both neurogenesis and neurodegeneration (14). Also, melatonin has been acclaimed in enhancing the efficacy of peripheral nerve regeneration in nerve defect of Wistar rats (15) which manifests its diverse therapeutic paradigm.
Concerning the neurodegenerative pathology, melatonin has been recognized to alleviating pathogenic mechanisms in AD (16) and its regulatory actions in controlling neurogenesis (17-19) with modulation of crucial signaling pathways (20) makes this remarkable indoleamine as one of the most efficient candidates in regulating the development and progression of many neurodegenerative disorders. Moreover, AD neuropathology in propinquity with pineal gland dysfunction and altered melatonin secretion has emerged to somehow discern the complexity of AD pathogenesis suggestive of impaired neurogenesis due to reduction in melatonin secretion (21). Therefore, owing to the multifactorial nature of AD which involves complex heterogeneous pathophysiological pathways, a broad-spectrum therapeutic paradigm should be considered. Melatonin stimulates early and late stages of neurodevelopment in the adult brain and significantly enhances memory and cognitive functions in aging, mild cognitive impairment (MCI) and AD. The conceivable factors mediating melatonin-induced adult neurogenesis are comprehensive (Figure 1). Therefore, melatonin pharmacotherapy strategy is pragmatic in combating the multispectral pathology of AD (22).
2. MELATONIN IN REGULATION OF THE PHYSIOLOGICAL HOMEOSTASIS OF NEUROGENIC NICHES
The insight that neurogenesis occurs in human brains throughout life led the understanding of learning and memory in a different direction (23, 24); contrary to the theory that neurogenesis was limited to embryonic development. This supervened as a complete scientific turn around as cognitive health of an organism is maintained by the capacity of hippocampal precursors to proliferate and differentiate.
2.1. Melatonin and neural stem cells.
Neural stem cells (NSCs) are immature progenitor cells in the CNS that are capable of self-replication and multipotent differentiation into neurons and glial cells. Adult mammalian neurogenesis occurs throughout life in the sub granular zone (SGZ) of dentate gyrus in the hippocampus and in the subventricular zone (SVZ) of the lateral ventricle (25-28). The newborn cells are incorporated into the extant circuitry of neurons which have been associated with the accretion of memory processes and cognitive functions (29) with phenomenal plasticity (30) thus boosting the total brain power. Therefore, the level of neurogenesis in the adult human hippocampus significantly contributes to the brain function (31).

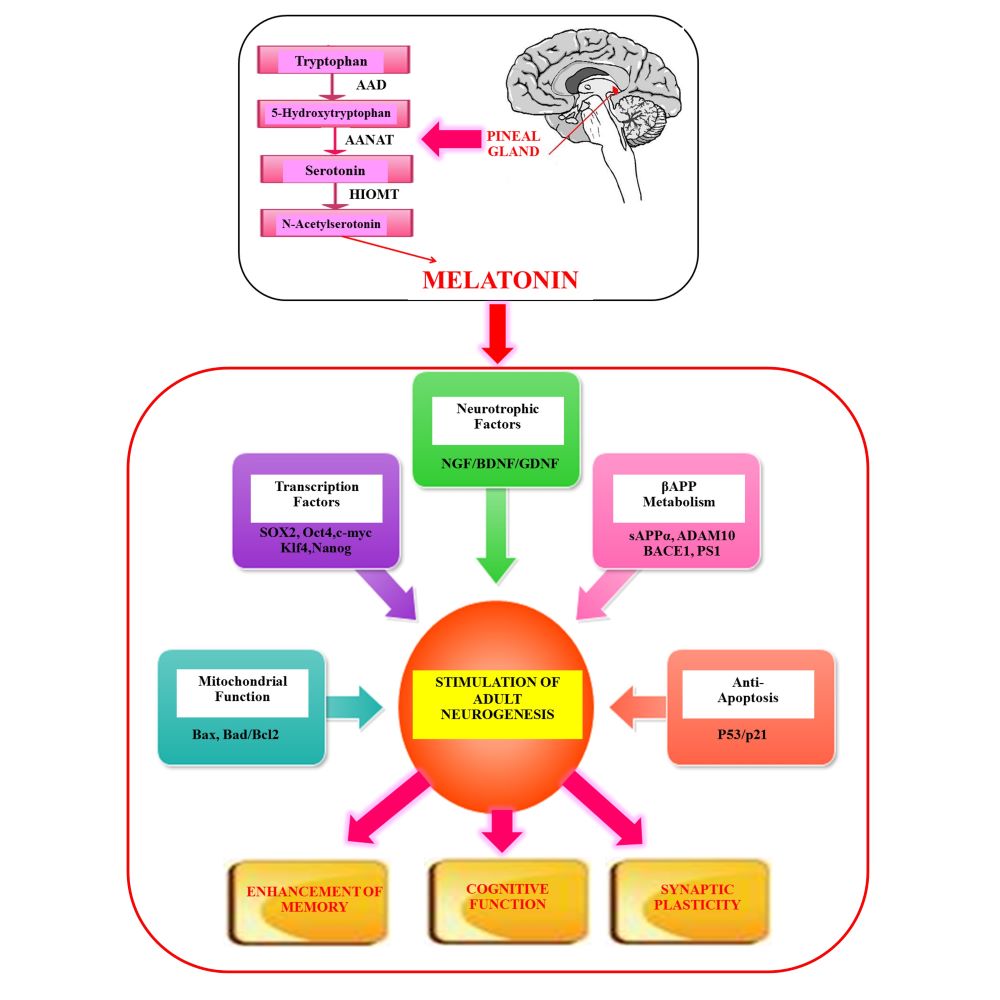
Fig. 1. Potential factors mediating melatonin-induced adult neurogenesis.
Melatonin stimulates adult neurogenesis by upregulating neurotrophic factors and transcription factor network. By regulating βAPP metabolism, metabolic homeostasis, stimulating anti-apoptotic and down regulating pro-apoptotic genes, melatonin boosts neurogenesis thus enhancing overall memory and cognitive functions. Abbreviations: βAPP, Beta-amyloid precursor protein.
Melatonin has been extensively investigated in regulating neurogenesis via multiple pathways (Figure 2). This indoleamine regulates the viability and guides directional differentiation of NSCs as evidenced in rat midbrain NSCs (32). It remarkably maintains and augments neurogenesis and has also been known to enhance the survival of new neurons, boost growth and maturation of dendrites and increase volume of the granular cell layer in the hippocampus of adult mice. Also, the regulatory action of melatonin in generation of new neurons in the dentate gyrus is evidenced by the enhancement in the volume of mossy fiber projections (9). Cultured C17.2 cells originally cloned from mouse cerebellar neural stem cells constitutively express several neurotrophic factors including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF), which play pivotal roles in neuronal development and differentiation (33). Physiological concentrations of melatonin increased neurite-like extensions and induced mRNA expression of the neural stem cell marker, nestin, the early neuronal marker beta-III-tubulin and the orphan nuclear receptor nurr1 in C17.2 cells (34). In addition, melatonin plays an important role in determining cell fate during neural commitment, thus promoting the differentiation of P19 mouse embryonic carcinoma cell line (P19) cells octamer-binding transcription factor 4 (Oct4+) SRY (sex determining region Y)-box 2, SOX2+) into neural stem cells via activation of the melatonin receptor subtype 1 (35). Interestingly, P19 cells originate from an embryo-derived teratocarcinoma, can differentiate into the three germ layers and is broadly used for examining the molecular mechanism of neurogenesis.

Fig. 2. Schematic illustration of melatonin mechanisms in regulating cellular proliferation and neuronal survival.
Melatonin regulates cellular proliferation and neuronal survival by both receptor dependent and independent mechanisms. It enhances the levels of β-catenin thereby regulating self-renewal, synaptic formation and plasticity, stimulates SOX2 levels which cross talks with cell cycle regulatory proteins and improves proliferation and by increasing SIRT1 blocks senescence. Melatonin regulates proliferation and differentiation of stem cells by enhancing stem cell markers. By instigating MEK1/ERK signaling pathway, melatonin 1) activates CaMKII and phosphorylates CREB thereby eliciting dendritogenesis 2) controls c-Myc which regulates cell cycle events and apoptosis (green arrows) 3) regulates mitochondrial function and blocks apoptosis, altogether promoting neuronal survival. Melatonin via PI3K/Akt signaling pathway; 1) stimulates Nrf2 and down regulates FOXO and procaspases thereby promoting neuronal survival 2) represses mTOR signaling pathway which in turn blocks tau hyperphosphorylation and regulates B-MYB which is involved in proliferation and senescence related phenomenon. Overall, it can be speculated that melatonin by regulating the above-mentioned mechanisms could possibly prevent neurodegenerative pathologies like aging and AD. SOX2, SRY (Sex determining region Y)-box 2; SIRT 1, silent mating type information regulation 2 homolog) 1; MEK1/ERK, Mitogen-activated protein kinase kinase 1 /extracellular signal-regulated kinases; CAMKII, Calcium/calmodulin-dependent kinase type II; CREB, cAMP-response element binding protein; c-Myc, master regulator of cell cycle entry and proliferative metabolism; PI3K/Akt, phosphatidylinositol-3-kinase/protein kinase B; Nrf2, nuclear factor erythroid 2-related factor 2); FOXO, Members of the class O of forkhead box transcription factors; mTOR, mammalian target of rapamycin; B-MYB, Myb-related protein B.
Melatonin stimulated NSC proliferation; increased the viability of cells, DNA synthesis (36) and significantly increased the number of neurospheres along with upregulation of multipotent stem cell markers (19). This indoleamine increased neural stem/progenitor cell proliferation in the dentate gyrus of rat pups (37) and increased the survival of neuronal progenitor cells in the dentate gyrus of adult mice (38, 39). Melatonin also stimulated the neural stem cells in adult mouse SVZ via its receptor activation by promoting bIII-Tubulin expression without affecting the levels of glial cell markers (17) and maturation of dendrites in new neurons formed in the dentate gyrus of mice (40).
The multiple actions of melatonin are modulated by many factors including Mitogen-activated protein kinase (MAPK)/Extracellular signal-regulated kinase (ERK) signaling pathway, histone acetylation, neurotrophic factors, transcription factors, and apoptotic genes (41). It has been reported that melatonin enhances NSC differentiation by increasing mitochondrial function (42). Growth factors are the main components for neurogenic induction in basic neural stem cell culture. A study by (18) compared the synergistic effects of epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) along with melatonin using neurosphere proliferation assay suggesting that the observed effects resulted due to activation of ERK/MAPK pathway. Another study in PC12 cell cultures also demonstrated that sub micromolar concentrations of melatonin exert a positive effect on neural differentiation involving the MEK/ERK1/2 signaling pathway (43). Importantly, melatonin membrane receptors are involved in the transition from NSCs and proliferative cells to the developmental stages as observed in the hippocampal neurogenic process of adult female mice (11) suggesting the pivotal involvement of melatonin in such mechanisms.
2.2. Melatonin acts via different transcription factors.
The specific transcription factors (Oct4, SOX2, c-Myc, and Krueppel-like factor 4 (Klf4)) dedifferentiate somatic cell lineages into a pluripotent state (44, 45). Melatonin induces mouse fibroblasts into induced pluripotent stem cells (iPSCs) possessing the same characteristics typical of embryonic stem cells (ESCs), including expression of the pluripotency markers Oct4, SOX2, and Nanog. Melatonin also promotes the expression of SOX2 and activates the phosphatidylinositol-3-kinase (PI3K)/Akt/ Nuclear factor erythroid 2–related factor 2 (Nrf2) signaling which authenticates its importance in survival and proliferation of NSCs (46). In addition, treatment with melatonin during the early stage of reprogramming downregulated the expression of the apoptosis-related genes p53 and p21 compared with the untreated controls (47). Melatonin administration also modulated primary cultured bovine NSCs isolated from the retinal neural layer by down regulating both p53 and p21 and increased reprogramming efficiency of N-iPS cell generation from primary cultured bovine NSCs via activation of ERK1/2 pathway (48). Melatonin not only enhanced the reprogramming efficiency but also significantly upregulated gene and protein expression of Nestin and Microtubule associated protein 2 (MAP2) in a receptor dependent manner (49) thus enhancing the proliferation and differentiation of iPSCs via activating ERK1/2 and (PI3K)/Akt signaling pathway.
Endogenous stem/progenitor cells have been investigated for enhancing cognition in the diseased brain (50). Neurons produced within canonical stem cell niches play a significant role in cognitive tasks (learning/memory) operated by specific neural systems (51, 52). Due to its remarkable protective potential and positive regulation of anti-aging mechanisms (53) and neurogenesis (14), melatonin has numerous applications in physiology and medicine pertaining to neurogenesis. Moreover, its involvement in regulation of important signaling pathways constitutes its role as a neurogenesis promoting agent (20). Nonetheless, a technical research patent on dendritogenesis and neuronal maturation has been drafted on the use of the melatonin molecule in stimulating neuronal maturation and dendritogenesis in adult mammals (54).
3. MELATONIN IN REGULATION OF NEUROGENESIS IN DIFFERENT PATHOLOGICAL CONDITIONS
Neurodegenerative diseases are associated with an exponential decrease in brain neurogenesis. The immature precursors of the CNS have self-renewal and multipotential differentiation abilities and their modulation with controlled pharmacological stimulation of these endogenous NSCs niches can be a promising therapeutic approach for many neurodegenerative disorders and other pathologies counteracting the neuronal loss. Melatonin has been accredited as a promising therapeutic approach for stimulating neurogenesis (55) by influencing proliferation and differentiation of NSCs in different physiological and pathological conditions (56).
3.1. Aging.
Maintaining healthy brain function in old age is an extremely critical aspect as both natural aging and age-related diseases are essentially comprised of pathological changes. The age-related decline in neurogenesis has been attributed to a decreased pool of NPCs because aging cells divide less in a given period of time, and the ones that do are more likely to re-enter the cell cycle within a day, both in vitro and in vivo (57). With advancing age there is an overall decrease in proliferation, growth factors, neuronal output and plasticity required for brain repair. NSCs undergo age-associated remodeling and dysfunction (58); therefore, therapeutic interventions targeting these niches could revitalize neurogenesis in the aged brain (59) .
Melatonin has been found to increase cell proliferation and survival in the hippocampus of aging mice (38, 39, 60). Its administration enhanced neurogenesis in old rats without modification of the total number of neurons and was able to reduce inflammation and apoptosis in the hippocampus (61, 62). As impaired neurogenesis and neurodegeneration promote the aging process in the nervous system, the recognition of melatonin as an anti-aging agent (53) with neurogenesis enhancing properties could have important therapeutic implications in aging (14) and neurodegenerative diseases (36).
3.2. Circadian dysrhythmia and sleep deprivation.
Circadian dysrhythmia has adverse impacts on body and mind. The circadian rhythm disorder "jet lag" disturbs hippocampal neurogenesis and spatial cognition, which represents morphological and functional adult brain plasticity. Moreover, altered circadian rhythms coincide with reduced nocturnal melatonin response in subjects with cognitive impairment (63). Melatonin promotes neurogenesis in circadian disruption (64), restores hippocampal neural precursor cell proliferation and prevents cognitive deficits induced by jet lag simulation in adult mice (65).
Sleep deprivation is associated with changes in hippocampal neurogenesis and cognitive processes causing a significant reduction in the number of new neurons in the SGZ, which may impair learning and memory performance (66). The prophylactic administration of melatonin increases the number of neural precursor cells in the adult SGZ. The possible mechanisms implicated in this induction involve circadian rhythm, melatonin receptors, and growth factors (67). Interestingly, melatonin promotes an increase in the tissue levels of B-cell lymphoma 2 (Bcl-2) in sleep-deprived animals. (68), using transgenic animals showed that Bcl-2 promotes neuronal maturation and hippocampal neurogenesis in the adult brain which gives a clear indicative of one of melatonin’s mechanism of action in enhancing neurogenesis. To add on, a recent study observed that melatonin administration enhanced SOX2+/5-bromo-2'-deoxyuridine (BrdU)+ cells in the SGZ of the sleep-deprived group (69). This study also explained the underlying mechanism according to which melatonin possibly modifies the expression of epigenetic mediators which in turn further regulate the proliferation of neural progenitor cells in the adult dentate gyrus under long-term sleep-deprived conditions.
3.3. Metabolic disorders.
Accumulating lines of evidence have marked that metabolic disorders like diabetes affect adult neurogenesis (70) where insulin/insulin like growth factor (IGF) signaling plays a pivotal role in modulating in NSC self-renewal, neurogenesis and cognition as evidenced in the CNS of mammalian models (71). Such involvement of disruptions in insulin/insulin receptor signaling, brain glucose uptake and tolerance in AD bridges the adult neurogenesis with energy metabolism and AD (72). Interestingly, it has also been evidenced in rodent and zebrafish models that diabetes impairs brain cell proliferation and differentiation thus affecting the overall brain cell survival (73). Type 2 is the most common form of diabetes reflected by peripheral blood hyperglycemia and one of the most prevalent risk factors for AD (74). Noteworthy, even the number and function of brain insulin receptors were found to be decreased clinically and in animal models of aging and AD (75).
In this frame of reference, melatonin prevented hyperglycemia induced detriments in brain of rat (76) and its receptors (Melatonin receptors 1 and 2) are associated pivotally in regulating glucose metabolism (77, 78). Recently, it has been established that melatonin prevents high glucose-induced apoptosis involving crucial signaling pathways like NF-κB, mTOR and Wnt in Schwann cells (79). Recently, it has been advocated that melatonin by restoring the brain insulin signaling substantially exerts neuroprotective effects on cognition in aged rats fed with high fat diet (80), herein, melatonin also prevented the increase in tau phosphorylation and amyloid beta (Aβ) accumulation in the hippocampus of rat brains.
Many previous studies have reported an inhibitory effect of melatonin on insulin release and contrary to this inference some studies have also demonstrated stimulatory effects. Therefore, based on the above congregated information there are some especially important aspects which needs to be mentioned and considered for elaborative investigations. Cohort studies have provided exceptional insights into such mechanisms where melatonin and its receptor (melatonin receptor 1B) play an important role in type 2 diabetes mellitus pathogenesis via directly acting on the β-cells (81) analyzing that the nocturnal melatonin secretion is independently and inversely associated with the insulin resistance (82). However, the melatonin induced decrement in glucose tolerance involved differential mechanisms during daytime and evening by either decreasing insulin release or by decreasing insulin sensitivity (83). Also, a human meta-analysis revealed that rs1387153, rs4753426, and rs10830963 variants of melatonin receptor 1B might serve as genetic biomarkers of gestational diabetes mellitus (84). As such both improved and impaired glucose tolerance has been reported after melatonin therapy. These discrepancies might have been alleged due to the different experimental models and species. One important reason is that melatonin is inversely correlated to activity/food intake in the human, as compared to species like nocturnal rodents. Furthermore, standards like the genetic background and time of the day play an important role in explaining the outcomes in human studies (85).
Although several studies are undergoing in order to investigate the underlying mechanisms, (86), demonstrated of late that melatonin augments proliferation and regulates differentiation of NSCs via autophagy in hyperglycemia. Moreover, an interesting study determined that co-treatment of bone marrow mesenchymal stem cells and melatonin improved the structural and functional efficiency of β-cell in streptozotocin diabetic male albino rats where melatonin by its own nature increased the vitality of stem cells (87). In a similar streptozotocin rat model, (88) , showed the therapeutic role of melatonin in protecting against diabetic central neuropathy, oxidative stress and neurodegeneration. In addition, this indoleamine enhanced the proliferation of NSCs derived from the brains of embryos from diabetic pregnant mice (89) which suggests that melatonin supplementation in diabetic pregnancy might prevent neural malformations in the offspring and when treated with Wnt-4 and retinoic acid, melatonin changed their morphology and enhanced expression of neural and glial cell markers (90).
The beneficial role of melatonin has also been demonstrated in mouse model of D-galactose-induced aging where it significantly restored the D-galactose-induced reduction of proliferating cells (Ki67-positive cells) and differentiating neuroblasts (doublecortin-positive neuroblasts) in the dentate gyrus (91) and attenuated the reduction of neurogenesis, synaptogenesis and the induction of astrogliosis induced by high-fat diet and streptozotocin in rat models (92).
3.4. Neurotoxic insults.
During embryonic stem cell self-renewal, growth factor deprivation and stress can lead to apoptosis and cell death (93). Dexamethasone is a synthetic glucocorticoid and exerts a neurotoxic action on rodent hippocampus. A study by (94) indicated a protective effect of melatonin on glucocorticoid neurotoxicity in the rat hippocampus. This indoleamine also protected hippocampal neurons from damage and reversed neurogenesis after chronic dexamethasone by activating BDNF and ERK1/2 cascades which indicates that melatonin possesses anti-stress and neurogenic actions (95). Apparently, melatonin regulates the cell viability, proliferation, and differentiation of NSCs from different brain regions (32, 36) and fosters neuroprotection against hypoxia (96), inflammation (46, 97) along with increasing the percentage of myelin basic protein (MBP)-positive cells in NSCs derived from mouse embryonic cortex (98).
Environmental stressors including irradiation have been shown to inhibit neurogenesis and are associated with the onset of cognitive impairments. Melatonin protects against radiation-induced impairment of neurogenesis and cognitive functions (99). Also, melatonin treatment recovers scopolamine-induced spatial learning and short-term memory impairments and restores or increases scopolamine-induced decrease of cell proliferation and neuroblast differentiation in the mouse dentate gyrus (100).
In a very recent study(101), demonstrated that melatonin prevented valproic acid induced spatial and non-spatial memory impairments and an abatement in hippocampal neurogenesis in male Sprague-dawley rats. Valproic acid is an anti-epileptic drug and it adversely affected hippocampal neurogenesis and memory. Whereas, methotrexate which is a chemotherapy agent is linked to cognitive deficits associated with decreased cell proliferation in the hippocampus in cancer patients. Interestingly, the neuroprotective potential of melatonin in diminishing the detrimental effects of methotrexate on memory and neurogenesis was validated (102). Besides, the neurotoxic effects of pesticide fenvalerate are well known. Melatonin significantly debilitated fenvalerate induced upregulation of pro-apoptotic genes like Bax, Fas, caspase 8, caspase 9, and caspase 3 and downregulation of anti-apoptotic gene Bcl-2. In addition, melatonin also prevented the decrease in the expression of neurogenesis-related genes (Distal-Less Homeobox 2 (Dlx2), Sonic hedgehog protein A precursor (Shha), Neurogenin1 (Ngn1), ELAV Like RNA Binding Protein 3 (Elavl3), and Glial fibrillary acidic protein (GFAP) in a zebrafish model (103).
On the other note, several studies have demonstrated that methamphetamine, a psychostimulant drug of abuse causes neurotoxic effects. Methamphetamine induces alterations in hippocampal neurogenesis (104, 105) and has been reported to disrupt development of neural progenitor cells in young adult nonhuman primates (106). In this context, melatonin administration attenuated methamphetamine induced inhibition of neurogenesis in the hippocampus of adult mouse (107). Melatonin also prevented a methamphetamine-induced reduction in cell proliferation (108) and abrogated dexamethasone-induced reductions in Ki-67 and alterations in G1-S phase cell cycle regulators in progenitor cells derived from the adult rat hippocampus (109).
To date, many different mechanisms have been proposed to cause dendritic spine dysfunction and loss in AD (110). Structural abnormalities in AD are characterized by decreased hippocampal volume and shrunken cortex. These structural changes are associated with diminished memory performance. The hilar neurons of the hippocampus integrate spatial memory and are lost in dementia. Melatonin increases dendrite maturation and complexity in new neurons formed in the dentate gyrus of adult mice (40) and repaired the loss of hippocampal dendrites by increasing calmodulin levels, activating Ca2+/calmodulin-dependent protein kinase II (CaMKII) thereby, eliciting dendritogenesis via protein kinase C (PKC) and its receptor activation (111).
Information from preclinical studies has shown that compounds with antidepressant effect possibly regulate adult hippocampal neurogenesis where enhancing the adult neurogenesis has been suggested to treat depression and anxiety (112). A human study in depressed subjects revealed that the decreased levels of melatonin corresponded to the diminished levels of neurotrophin-3 (NT-3), BDNF and NGF when compared to healthy controls (113). In this context, melatonin also possesses antidepressant like effects (114) along with its ability to regulate adult hippocampal neurogenesis.
Furthermore, in combination with exercise, melatonin increased the number of BrdU-positive Nestin-expressing endogenous neural stem cells in a rat model of spinal cord injury (115) and potentiated running wheel-induced hippocampal neurogenesis by enhancing neuronal survival suggesting that the combination of physical exercise and melatonin may be an effective treatment for diseases affecting the hippocampus neurogenesis (116). The fact that agents promoting BDNF signaling might be effective in treating and preventing AD (117) supports the probability of melatonin as an anti-AD compound based on the study by (100) which showed that melatonin treatment increased BDNF and tropomyosin receptor kinase B (TrkB) expressions in the mouse dentate gyrus.
Ischemia affects the neurovascular complex of the AD brain by altering the cholinergic system in concomitance with vascular pathology as observed in mice models (118). Whereas, melatonin pretreatment increased survival of mesenchymal stem cells in vitro and reduced apoptosis after transplantation into ischemic rat brain (119) and played a role in protecting the cholinergic system (120). Moreover, treatment with melatonin after stroke dramatically enhanced endogenous neurogenesis and cell proliferation in the peri-infarct regions of mice by activating melatonin receptors (6) and has been beneficial for treating cerebral infarction (36). Melatonin also upregulated GDNF expression (121) and enhanced glial cell survival in case of cerebral ischemia (122) which depicts yet another therapeutic mechanism of melatonin action in alleviating pathological mechanisms in AD.
3.5. Melatonin analogs in inducing neurogenesis in pathological conditions.
Neurons produced within canonical stem cell niches play a significant role in cognitive tasks (51, 52); therefore, endogenous stem/progenitor cells have been investigated as a way of enhancing cognition in the diseased brain (50). Cognitive health of an organism is maintained by the capacity of hippocampal neurogenesis which plays an important role in ameliorating the deficits in neurodegenerative diseases.
The immunological mechanisms in the brain have diverse interactions with the AD pathogenesis where aggregated proteins trigger release of inflammatory mediators, which are responsible for disease progression and severity (123). Interestingly, niche-derived inflammatory signals induce quiescence as observed in aging brain which is accompanied by a systematic drop in the NSCs which is considered as a rescue response that prevents exhaustive depletion of these resting cells (124). Systemic neuroinflammation induced enhancement of proinflammatory cytokines pose negative impact on neurogenesis and on the cognitive reserve as observed in progressive aging and neurodegenerative disorders like AD (125, 126). An additional mechanism through which inflammation could disrupt cognitive function is through altering levels of neurotrophic factors in the brain such as BDNF etc. which are known to be critically involved in supporting memory formation, neurogenesis and LTP (127). In this context, melatonin has been known to immensely affect the immune cells functioning. As the pro-/anti-inflammatory aspect concerning neurogenesis extends to the role of macrophage/microglia polarization (128) and the capability of melatonin of favoring polarization, i.e., anti- vs. proinflammatory behavior (129) may be of substantial relevance. Our previous study demonstrated how melatonin significantly attenuated the pro-inflammatory cytokines and enhanced BDNF in the hippocampus of aged mouse brain (97). Moreover, melatonin also reduced Aβ42 induced NFκB activation in neuroblastoma cell cultures (130) which gives a clear indication of its anti-inflammatory role in an aging and diseased brain.
Investigations have manifested that melatonin-based compounds promote the differentiation of NSCs into neuronal phenotype (131). N-Acetylserotonin is a precursor of melatonin involved in its biosynthesis. Not only this compound stimulates proliferation of neural progenitor cells in the hippocampus but also prevents sleep deprivation induced harmful effects (132, 133). Melatonin pretreatment and treatment with its metabolite N(1)-acetyl-N(2)-formyl-5-methoxykynuramine (AFMK), significantly ameliorated the radiation-induced decline in the anti-doublecortin and Ki-67 positive cells in mouse brain (134, 135) and since oxidative stress is reported to be implicated in impaired neurogenesis, it is likely that antioxidants such as melatonin and its metabolites could restore or minimize cellular death in the hippocampal dentate gyrus (99). Agomelatine is a novel antidepressant acting as a melatonergic receptor agonist and serotonergic (5-HT (2C)) receptor antagonist. In adult rats, chronic agomelatine treatment enhanced cell proliferation and neurogenesis in the ventral hippocampus. Agomelatine increased the ratio of mature vs. immature neurons and enhanced neurite outgrowth of granular cells, suggesting an acceleration of maturation. The influence of agomelatine on maturation and survival was accompanied by a selective increase in the levels of BDNF (136) and it also increased hippocampal neurogenesis and ameliorated apoptosis in the hippocampus of rat brains exposed to stress (137). Previous studies have demonstrated that piromelatine (a melatonin and serotonin 5-HT1A and 5-HT1D agonist) exerts an antidepressant activity in rodent models of acute stress and improves cognitive impairments in a rat model of AD. Piromelatine ameliorates memory deficits in rats and this effect may be mediated by restoring hippocampal BDNF, cAMP-response element binding protein (CREB), and cytogenesis deficits (138). Recently, it has been shown that the melatonin analog 2-(2-(5-methoxy-1 H-indol-3-yl) ethyl)-5-methyl-1,3,4-oxadiazole (IQM316) induced hippocampal neurogenesis with concurrently preserving previously attained memories in adult mice (139).
4. ROLE OF MELATONIN IN REGULATING NEUROGENESIS VIA BETA AMYLOID PRECURSOR PROTEIN (βAPP) PROCESSING PATHWAYS (Figure 3)
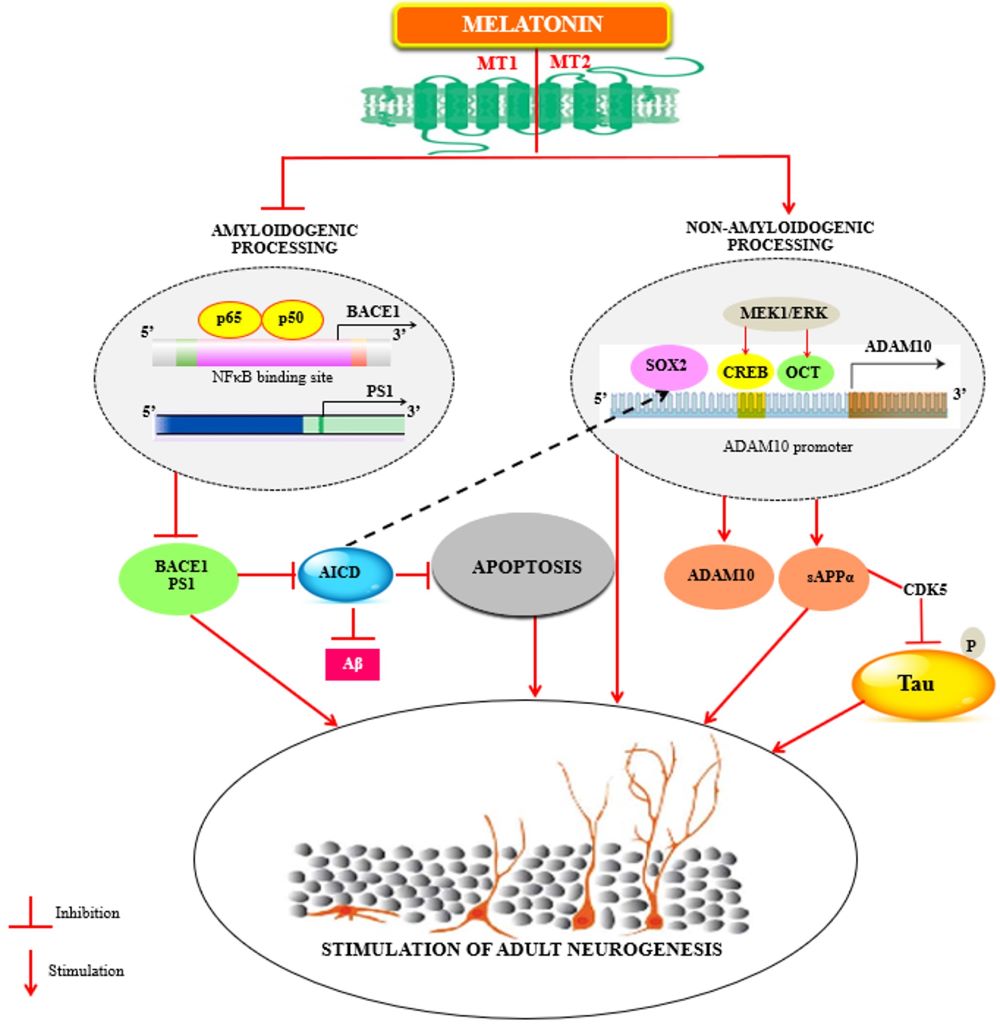
Fig. 3. Melatonin regulates adult neurogenesis by regulating βAPP metabolism.
As increase in amyloidogenic cleavage of βAPP triggers an overproduction of AICD, which not only initiates the apoptotic process but also inhibits SOX2 expression at the transcriptional level (black lines) further leading to a diminution of ADAM10 transcription (for review; Sarlak et.al, 2016) subsequently impairing neurogenesis. Melatonin regulates the non-pathological βAPP homeostasis by stimulating the non-amyloidogenic processing and down regulating the amyloidogenic processing of βAPP. By decreasing the mRNA levels of both BACE1 (via NFκB) and PS1, melatonin might preclude the production of AICD and Aβ which implies its anti-apoptotic effects and simultaneous regulation of Aβ induced alterations in neurogenesis. Parallelly melatonin enhances sAPPα and stimulates ADAM10 levels by increasing its promoter transactivation. Increase in sAPPα down regulates CDK5 induced tau hyperphosphorylation. By stimulating SOX2 levels which are involved in transcriptional regulation of ADAM10, melatonin thereby augments the neurogenic processes. AICD, amyloid precursor protein intracellular domain; SOX2, SRY (Sex determining region Y)-box 2; ADAM10, A disintegrin and metalloproteinase domain-containing protein 10; BACE1, Beta-site amyloid precursor protein cleaving enzyme 1; NFκB, Nuclear factor kappa-light-chain-enhancer of activated B cells; PS1, Presenilin 1; AβAmyloid beta -peptide; sAPPα, Soluble amyloid precursor protein alpha; CDK5, Cyclin-dependent kinase 5.
The neurodegenerative events of AD progress throughout the temporal, frontal and parietal lobes (140) of the brain and it has been well known that hippocampus which is affected during early stages of AD is one of the two neurogenic niches of the adult brain (141). Therefore, impairment of neurogenesis is quite related to the disease progression itself. Beta amyloid precursor protein (βAPP) the precursor of amyloid beta (Aβ) is important for neuron generation, differentiation and neural migration (142, 143) as the embryonic expression of βAPP peaks during neuronal differentiation and neurite outgrowth period (144, 145). The studies performed on transgenic animals expressing mutant βAPP demonstrated decreased neurogenesis in adult brains (146-149). Noteworthy is that melatonin levels are decreased in blood and cerebrospinal fluid (CSF) of AD patients and this reduction runs parallel with the progression of AD pathogenesis (150). Accumulation of hyperphosphorylated and aggregated tau reduces adult neurogenesis (151) and melatonin ameliorated Aβ and wortmannin-induced tau hyperphosphorylation (152, 153), neurodegeneration and memory deficits in mouse hippocampus. In vitro studies demonstrated that melatonin concentration dependently prevented Aβ induced apoptotic mechanisms in neuronal cell cultures due to its anti-amyloidogenic properties (154, 155). Interestingly, melatonin reduced Aβ accumulation in hippocampus and entorhinal cortex and prevented the cognitive impairment in APP + presenilin1 (PS1) double transgenic (Tg) mouse model (156). Diminished α-secretase activity leads to AD-related pathology (157), whereas sAPPα is persistently involved in specific regulation of gene expression in hippocampus as demonstrated in rat experimental models (158).
4.1. Nonamyloidogenic pathway.
In this context, the first demonstration that melatonin upregulates the nonamyloidogenic constitutive and regulated A Disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) and ADAM metallopeptidase domain 17 (ADAM17) proteases which enhances the neuroprotective sAPPα through melatonin receptor was provided by (159). Additionally, melatonin also prevented Aβ42 induced reduction in ADAM10 protein expression via its receptor activation involving Pin1/GSK3β/NF-κB pathway (130). An increase in ADAM10 activity shifts the balance of βAPP processing toward soluble α-APP (sAPPα) (160) and protects the brain from amyloid deposition and tau pathology in the brain (161). As such the levels of ADAM10 are reduced in AD patients (162) and blocking sAPPα secretion or down regulating βAPP synthesis impairs the proliferation of epidermal growth factor responsive cells, which leads to decrease in number of neuronal progenitor cells in SVZ (163, 164). Whereas, both βAPP and PS1, the catalytic core of one of the enzymes that cleaves βAPP, play a role in regulation of neurogenesis during development and postnatally and how βAPP regulates neurogenesis in physiological conditions and in AD has been reviewed extensively (165). Neurogenesis declines with aging and sAPPα rescues age associated decline in neural progenitor cell proliferation (2). sAPPα protects neurons and promotes neurogenesis (166, 167), enhances neurite outgrowth and presynaptic bouton density in differentiating NPCs isolated from both embryonic and adult brains (168, 169) and promotes neuronal differentiation. sAPPα protects neurons and promotes neurogenesis, possibly mediated by its ability to prevent over activation of cyclin-dependent kinase 5 (CDK5) and tau hyperphosphorylation (170).
sAPPα binds to SVZ progenitor cells expressing the EGF receptor and increase their proliferation (171) along with eliciting neuroprotection, synaptic plasticity, memory formation, neurogenesis, and neuritogenesis, while reducing amyloid and tau pathology in the brain (161). Melatonin enhances the α-secretase processing of βAPP and increases sAPPα levels (159) and increases reprogramming efficiency of NSC-derived pluripotent stem cells (N-iPS) cell generation (48).
4.2. Amyloidogenic pathway.
Conversely to sAPPα function, amyloid precursor protein intracellular domain (AICD) has been shown to be a negative regulator of proliferation in neural progenitor cells (NPCs). AICD negatively regulates the transcription of the epidermal growth factor receptor (EGFR), a receptor that drives NPCs proliferation (149, 172). Adult neurogenesis is functionally associated with AD-like neurodegeneration (173) as the increase in amyloidogenic processing of βAPP triggers an overproduction of AICD which has been shown to decrease hippocampal progenitor cell proliferation and survival in transgenic mice (174) and this increase in AICD expression after a certain point leads to apoptosis simultaneously inhibiting SOX2 expression at a transcriptional level which subsequently leads to an impairment of neurogenesis together with neurodegeneration thereby reducing ADAM10 transcription and sAPPα secretion (175, 176) Melatonin enhances the expression of pluripotent genes-including (POU Class 5 Homeobox 1 (Pou5f1), SOX2, Klf4, c-Myc, Nanog, Lin28a, and surface marker proteins from embryonic stem-like cells from rabbit blastocysts (177).
Although it has been shown that β-Site amyloid precursor protein cleaving enzyme 1 (BACE1) inhibition improves cognitive functions and reverse amyloid deposition in an 5xFAD mouse model (178), but due to its involvement in regulation of adult hippocampal neurogenesis the complete therapeutic inhibition of BACE1 activity would certainly dysregulate neurogenesis in adult mouse hippocampus (179). Therefore, it would be more reasonable to opt for partial inhibition of BACE1 activity for therapeutic purposes. Whereas, PS1 is the catalytic core of the aspartyl protease γ-secretase which is also involved in regulation of the differentiation of adult NPCs (180). It has been demonstrated that knockdown of PS1 in NPCs in the sub granular layer of dentate gyrus induces learning impairments (181). As PS1 effect on neurogenesis is mediated via β-catenin phosphorylation and notch signaling, it is therefore speculated that inhibiting PS1 completely would affect the neurogenesis processes. Considering the above parameters, it would be meaningful to assess the therapeutic potential of melatonin. In this context, it has been shown that melatonin also down regulates the amyloidogenic processing of βAPP by regulating both β- and γ-secretases at the transcriptional level (182) without complete inhibition. Moreover, melatonin has been shown to reverse the age dependent alteration of βAPP cleaving secretases in hippocampus of aged mouse (183). Overall, it could be speculated how this multifunctional indoleamine could possibly regulate neurogenesis in pathological conditions like AD.
5. MELATONIN DOSES AND CONCENTRATIONS EMPLOYED IN VITRO WITH THE PRESUMPTIVE THERAPEUTIC DOSES IN HUMANS
Therapeutic effects of melatonin have been reported in large number of disorders of different etiologies such as neurodegenerative disorders, cardiovascular diseases, sleep disorders, psychiatric disorders etc. Regarding its production nearly 80% of the melatonin is synthesized at night, with serum concentrations varying between (80-120 pg/ml) whereas low serum concentrations (10-20 pg/ml) are present during the daylight hours. In humans, 1-5mg has been considered as an average dosage range where the bioavailability also depends upon the route of administration (184, 185). An important retrospective study on the efficacy of melatonin in AD patients revealed that 9 mg gelatin melatonin capsules p.o. daily at bedtime for 22 to 35 months stabilized the sleep and cognitive disorders (186). However, melatonin as high as 20mg have also been used in malignancies. Several in vitro studies have shown that the pharmacologic concentrations of melatonin is 1 mM, but the physiological concentration in humans is about 70 pM.
Furthermore, on investigating in different animal models, melatonin at subacute dose of 4 mg/kg/day for a continuous period of 29 days improved neuronal survival and enhanced neurogenesis in a stroke model of mice (187). Another study in adult Balb/C mice demonstrated that administration of oral melatonin at a dose of 10 mg/kg of body weight per day enhanced neurogenesis (188). Similarly, in the dentate gyrus of adult C57BL/6 mice in vivo, exogenous melatonin (8 mg/kg) also increased the survival of neuronal progenitor cells and post-mitotic immature neurons (189). The administration of both physiological (5-10 microg/kg) and pharmacological (20-320 microg/kg) doses produced different effects on sleep efficiency in three species of diurnal nonhuman primates as these could possibly serve as adequate animal models for studying the mechanisms of melatonin's action on such disorders (190).
Referring to the brain, a number of evidence which have been accumulated from studies on various neurodegeneration models and clinical reports support the use of melatonin for the preventive treatment of major neurodegenerative disorders like AD, Parkinson disease, Huntington's disease and Amyotrophic Lateral Sclerosis (191). Additional studies are required to identify the specific therapeutic concentrations and the dose-response relationships to test the clinical efficacy of melatonin supplementation (192) in various disorders in order to extend the use of melatonin in clinical practice, prevention and treatment. However, regarding the therapeutic aftermath of melatonin use in enhancing neurogenesis in humans need substantive clinical evidence before any precise recommendations can further be formulated.
6. SUMMARY
The complex circuitry between neuroregenerative and neurodegenerative processes revolves around the mechanisms interlinked between the parameters coupled with neurogenesis. The homeostatic balance between these variables goes awry in degenerative diseases like AD. The phenomenal regulation of neurogenesis by melatonin reveals a remarkable regulatory network of this indoleamine in governing not only the molecular events which occur during the development of the disease but also regulating stem cells which have the potential to repair brain damage and may aid in developing novel strategies for neurodegenerative disease therapy.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the support from Chulabhorn Graduate Institute. We thank Ms Parichart Boontem and Ms Juthatip Niyomrat for technical assistance.
AUTHORSHIP
MS drafted the first version of manuscript, prepared figures, AS and PG revised the manuscript critically and PG approved it.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
REFERENCES
Lobato RD (2008) Historical vignette of Cajal's work "Degeneration and regeneration of the nervous system" with a reflection of the author. Neurocirugia (Astur.) 19: 456-468.
Demars MP, Hollands C, Zhao Kda T, Lazarov O (2013) Soluble amyloid precursor protein-alpha rescues age-linked decline in neural progenitor cell proliferation. Neurobiol. Aging 34: 2431-2440. doi: 10.1016/j.neurobiolaging.2013.04.016.
Walsh CE,Hitchcock PF (2017) Progranulin regulates neurogenesis in the developing vertebrate retina. Dev. Neurobiol. 77: 1114-1129. doi: 10.1002/dneu.22499.
Moreno-Jimenez EP, Flor-Garcia M, Terreros-Roncal J, Rabano A, Cafini F, Pallas-Bazarra N, Avila J, Llorens-Martin M (2019) Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease. Nat. Med. 25: 554-560. doi: 10.1038/s41591-019-0375-9.
Hollands C, Bartolotti N, Lazarov O (2016) Alzheimer's disease and hippocampal adult neurogenesis; exploring shared mechanisms. Front. Neurosci. 10: 178. doi: 10.3389/fnins.2016.00178.
Chern CM, Liao JF, Wang YH, Shen YC (2012) Melatonin ameliorates neural function by promoting endogenous neurogenesis through the MT2 melatonin receptor in ischemic-stroke mice. Free Radic. Biol. Med. 52: 1634-1647. doi: 10.1016/j.freeradbiomed.2012.01.030.
Hossain MF, Uddin MS, Uddin GMS, Sumsuzzman DM, Islam MS, Barreto GE, Mathew B, Ashraf GM (2019) Melatonin in Alzheimer's disease: A latent endogenous regulator of neurogenesis to mitigate Alzheimer's neuropathology. Mol. Neurobiol. 56: 8255-8276. doi: 10.1007/s12035-019-01660-3.
El-Sherif Y, Tesoriero J, Hogan MV, Wieraszko A (2003) Melatonin regulates neuronal plasticity in the hippocampus. J. Neurosci. Res. 72: 454-460. doi: 10.1002/jnr.10605.
Ramirez-Rodriguez GB, Olvera-Hernandez S, Vega-Rivera NM, Ortiz-Lopez L (2018) Melatonin Influences Structural Plasticity in the Axons of Granule Cells in the Dentate Gyrus of Balb/C Mice. Int. J. Mol. Sci. 20: doi: 10.3390/ijms20010073.
Toda T,Gage FH (2018) Review: adult neurogenesis contributes to hippocampal plasticity. Cell Tissue Res. 373: 693-709. doi: 10.1007/s00441-017-2735-4.
Ortiz-Lopez L, Perez-Beltran C, Ramirez-Rodriguez G (2016) Chronic administration of a melatonin membrane receptor antagonist, luzindole, affects hippocampal neurogenesis without changes in hopelessness-like behavior in adult mice. Neuropharmacology 103: 211-221. doi: 10.1016/j.neuropharm.2015.11.030.
Ng KY, Leong MK, Liang H, Paxinos G (2017) Melatonin receptors: distribution in mammalian brain and their respective putative functions. Brain Struct. Funct. 222: 2921-2939. doi: 10.1007/s00429-017-1439-6.
Motta-Teixeira LC, Machado-Nils AV, Battagello DS, Diniz GB, Andrade-Silva J, Silva S, Jr., Matos RA, do Amaral FG, Xavier GF, Bittencourt JC, Reiter RJ, Lucassen PJ, Korosi A, Cipolla-Neto J (2018) The absence of maternal pineal melatonin rhythm during pregnancy and lactation impairs offspring physical growth, neurodevelopment, and behavior. Horm. Behav. 105: 146-156. doi: 10.1016/j.yhbeh.2018.08.006.
Sarlak G, Jenwitheesuk A, Chetsawang B, Govitrapong P (2013) Effects of melatonin on nervous system aging: neurogenesis and neurodegeneration. J. Pharmacol. Sci. 123: 9-24. doi: 10.1254/jphs.13r01sr.
Salehi M, Naseri-Nosar M, Ebrahimi-Barough S, Nourani M, Khojasteh A, Farzamfar S, Mansouri K, Ai J (2018) Polyurethane/gelatin nanofibrils neural guidance conduit containing platelet-rich plasma and melatonin for transplantation of schwann cells. Cell Mol. Neurobiol. 38: 703-713. doi: 10.1007/s10571-017-0535-8.
Shukla M, Govitrapong P, Boontem P, Reiter RJ, Satayavivad J (2017) Mechanisms of melatonin in alleviating Alzheimer's disease. Curr. Neuropharmacol. 15: 1010-1031. doi: 10.2174/1570159X15666170313123454.
Sotthibundhu A, Phansuwan-Pujito P, Govitrapong P (2010) Melatonin increases proliferation of cultured neural stem cells obtained from adult mouse subventricular zone. J. Pineal Res. 49: 291-300. doi: 10.1111/j.1600-079X.2010.00794.x.
Sotthibundhu A, Ekthuwapranee K, Govitrapong P (2016) Comparison of melatonin with growth factors in promoting precursor cells proliferation in adult mouse subventricular zone. EXCLI J. 15: 829-841. doi: 10.17179/excli2016-606.
Tocharus C, Puriboriboon Y, Junmanee T, Tocharus J, Ekthuwapranee K, Govitrapong P (2014) Melatonin enhances adult rat hippocampal progenitor cell proliferation via ERK signaling pathway through melatonin receptor. Neuroscience 275: 314-321. doi: 10.1016/j.neuroscience.2014.06.026.
Shukla M, Chinchalongporn V, Govitrapong P, Reiter RJ (2019) The role of melatonin in targeting cell signaling pathways in neurodegeneration. Ann. N. Y. Acad. Sci. 1443: 75-96. doi: 10.1111/nyas.14005.
Song J (2019) Pineal gland dysfunction in Alzheimer's disease: relationship with the immune-pineal axis, sleep disturbance, and neurogenesis. Mol. Neurodegener. 14: 28. doi: 10.1186/s13024-019-0330-8.
Daulatzai MA (2016) Pharmacotherpy and Alzheimer's Disease: The M-drugs (melatonin, minocycline, modafinil, and memantine) approach. Curr. Pharm. Des. 22: 2411-2430. doi: 10.2174/1381612822666160203142111.
Altman J (1962) Are new neurons formed in the brains of adult mammals? Science 135: 1127-1128. doi: 10.1126/science.135.3509.1127.
Altman J,Das GD (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 124: 319-335. doi: 10.1002/cne.901240303.
Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat. Med. 4: 1313-1317. doi: 10.1038/3305.
Kempermann G (2002) Why new neurons? Possible functions for adult hippocampal neurogenesis. J. Neurosci. 22: 635-638.
Ming GL,Song H (2005) Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 28: 223-250. doi: 10.1146/annurev.neuro.28.051804.101459.
Ming GL,Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70: 687-702. doi: 10.1016/j.neuron.2011.05.001.
Meerlo P, Mistlberger RE, Jacobs BL, Heller HC, McGinty D (2009) New neurons in the adult brain: the role of sleep and consequences of sleep loss. Sleep Med. Rev. 13: 187-194. doi: 10.1016/j.smrv.2008.07.004.
Goncalves JT, Schafer ST, Gage FH (2016) Adult Neurogenesis in the hippocampus: from stem cells to behavior. Cell 167: 897-914. doi: 10.1016/j.cell.2016.10.021.
Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisen J (2013) Dynamics of hippocampal neurogenesis in adult humans. Cell 153: 1219-1227. doi: 10.1016/j.cell.2013.05.002.
Kong X, Li X, Cai Z, Yang N, Liu Y, Shu J, Pan L, Zuo P (2008) Melatonin regulates the viability and differentiation of rat midbrain neural stem cells. Cell. Mol. Neurobiol. 28: 569-579. doi: 10.1007/s10571-007-9212-7.
Niles LP, Armstrong KJ, Rincon Castro LM, Dao CV, Sharma R, McMillan CR, Doering LC, Kirkham DL (2004) Neural stem cells express melatonin receptors and neurotrophic factors: colocalization of the MT1 receptor with neuronal and glial markers. BMC Neurosci. 5: 41. doi: 10.1186/1471-2202-5-41.
Sharma R, Ottenhof T, Rzeczkowska PA, Niles LP (2008) Epigenetic targets for melatonin: induction of histone H3 hyperacetylation and gene expression in C17.2 neural stem cells. J. Pineal Res. 45: 277-284. doi: 10.1111/j.1600-079X.2008.00587.x.
Chen X, Li X, Du Z, Shi W, Yao Y, Wang C, He K, Hao A (2014) Melatonin promotes the acquisition of neural identity through extracellular-signal-regulated kinases 1/2 activation. J. Pineal Res. 57: 168-176. doi: 10.1111/jpi.12153.
Moriya T, Horie N, Mitome M, Shinohara K (2007) Melatonin influences the proliferative and differentiative activity of neural stem cells. J. Pineal Res. 42: 411-418. doi: 10.1111/j.1600-079X.2007.00435.x.
Kim MJ, Kim HK, Kim BS, Yim SV (2004) Melatonin increases cell proliferation in the dentate gyrus of maternally separated rats. J. Pineal Res. 37: 193-197. doi: 10.1111/j.1600-079X.2004.00157.x.
Ramirez-Rodriguez G, Klempin F, Babu H, Benitez-King G, Kempermann G (2009) Melatonin modulates cell survival of new neurons in the hippocampus of adult mice. Neuropsychopharmacology 34: 2180-2191. doi: 10.1038/npp.2009.46.
Ramirez-Rodriguez G, Vega-Rivera NM, Benitez-King G, Castro-Garcia M, Ortiz-Lopez L (2012) Melatonin supplementation delays the decline of adult hippocampal neurogenesis during normal aging of mice. Neurosci. Lett. 530: 53-58. doi: 10.1016/j.neulet.2012.09.045.
Dominguez-Alonso A, Ramirez-Rodriguez G, Benitez-King G (2012) Melatonin increases dendritogenesis in the hilus of hippocampal organotypic cultures. J. Pineal Res. 52: 427-436. doi: 10.1111/j.1600-079X.2011.00957.x.
Chu J, Tu Y, Chen J, Tan D, Liu X, Pi R (2016) Effects of melatonin and its analogues on neural stem cells. Mol. Cell. Endocrinol. 420: 169-179. doi: 10.1016/j.mce.2015.10.012.
Mendivil-Perez M, Soto-Mercado V, Guerra-Librero A, Fernandez-Gil BI, Florido J, Shen YQ, Tejada MA, Capilla-Gonzalez V, Rusanova I, Garcia-Verdugo JM, Acuna-Castroviejo D, Lopez LC, Velez-Pardo C, Jimenez-Del-Rio M, Ferrer JM, Escames G (2017) Melatonin enhances neural stem cell differentiation and engraftment by increasing mitochondrial function. J. Pineal Res. 63: e12415. doi: 10.1111/jpi.12415.
Liu Y, Zhang Z, Lv Q, Chen X, Deng W, Shi K, Pan L (2016) Effects and mechanisms of melatonin on the proliferation and neural differentiation of PC12 cells. Biochem. Biophys. Res. Commun. 478: 540-545. doi: 10.1016/j.bbrc.2016.07.093.
Takahashi K,Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663-676. doi: 10.1016/j.cell.2006.07.024.
Takahashi K,Yamanaka S (2016) A decade of transcription factor-mediated reprogramming to pluripotency. Nat. Rev. Mol. Cell Biol. 17: 183-193. doi: 10.1038/nrm.2016.8.
Song J, Kang SM, Lee KM, Lee JE (2015) The protective effect of melatonin on neural stem cell against LPS-induced inflammation. Biomed. Res. Int. 2015: 854359. doi: 10.1155/2015/854359.
Gao S, Wang ZL, Di KQ, Chang G, Tao L, An L, Wu FJ, Xu JQ, Liu YW, Wu ZH, Li XY, Gao S, Tian JH (2013) Melatonin improves the reprogramming efficiency of murine-induced pluripotent stem cells using a secondary inducible system. J. Pineal Res. 55: 31-39. doi: 10.1111/jpi.12047.
Bai C, Li X, Gao Y, Yuan Z, Hu P, Wang H, Liu C, Guan W, Ma Y (2016) Melatonin improves reprogramming efficiency and proliferation of bovine-induced pluripotent stem cells. J. Pineal Res. 61: 154-167. doi: 10.1111/jpi.12334.
Shu T, Wu T, Pang M, Liu C, Wang X, Wang J, Liu B, Rong L (2016) Effects and mechanisms of melatonin on neural differentiation of induced pluripotent stem cells. Biochem. Biophys. Res. Commun. 474: 566-571. doi: 10.1016/j.bbrc.2016.04.108.
Bordey A (2014) Endogenous stem cells for enhancing cognition in the diseased brain. Front. Neurosci. 8: 98. doi: 10.3389/fnins.2014.00098.
Lepousez G, Valley MT, Lledo PM (2013) The impact of adult neurogenesis on olfactory bulb circuits and computations. Annu. Rev. Physiol. 75: 339-363. doi: 10.1146/annurev-physiol-030212-183731.
Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH (2014) Regulation and function of adult neurogenesis: from genes to cognition. Physiol. Rev. 94: 991-1026. doi: 10.1152/physrev.00004.2014.
Hardeland R (2013) Melatonin and the theories of aging: a critical appraisal of melatonin's role in antiaging mechanisms. J. Pineal Res. 55: 325-356. doi: 10.1111/jpi.12090.
Soto-Vazquez R, Labastida-Lopez C, Romero-Castello S, Benitez-King G, Parra-Cervantes P (2016) Stimulation of dendrogenesis and neural maturation in adult mammals. Pharm. Pat. Anal. 5: 183-193. doi: 10.4155/ppa.15.43.
Herrera-Arozamena C, Marti-Mari O, Estrada M, de la Fuente Revenga M, Rodriguez-Franco MI (2016) Recent advances in neurogenic small molecules as innovative treatments for neurodegenerative diseases. molecules 21: 1165. doi: 10.3390/molecules21091165.
Yu X, Li Z, Zheng H, Ho J, Chan MT, Wu WK (2017) Protective roles of melatonin in central nervous system diseases by regulation of neural stem cells. Cell Prolif. 50: e12323. doi: 10.1111/cpr.12323.
Stoll EA, Habibi BA, Mikheev AM, Lasiene J, Massey SC, Swanson KR, Rostomily RC, Horner PJ (2011) Increased re-entry into cell cycle mitigates age-related neurogenic decline in the murine subventricular zone. Stem Cells 29: 2005-2017. doi: 10.1002/stem.747.
Apple DM, Solano-Fonseca R, Kokovay E (2017) Neurogenesis in the aging brain. Biochem. Pharmacol. 141: 77-85. doi: 10.1016/j.bcp.2017.06.116.
Katsimpardi L,Lledo PM (2018) Regulation of neurogenesis in the adult and aging brain. Curr. Opin. Neurobiol. 53: 131-138. doi: 10.1016/j.conb.2018.07.006.
Ramirez-Rodriguez G, Gomez-Sanchez A, Ortiz-Lopez L (2014) Melatonin maintains calcium-binding calretinin-positive neurons in the dentate gyrus during aging of Balb/C mice. Exp. Gerontol. 60: 147-152. doi: 10.1016/j.exger.2014.10.014.
Kireev RA, Cuesta S, Vara E, Tresguerres JA (2011) Effect of growth hormone and melatonin on the brain: from molecular mechanisms to structural changes. Horm. Mol. Biol. Clin. Investig. 7: 337-350. doi: 10.1515/HMBCI.2011.115.
Kireev RA, Vara E, Tresguerres JA (2013) Growth hormone and melatonin prevent age-related alteration in apoptosis processes in the dentate gyrus of male rats. Biogerontology 14: 431-442. doi: 10.1007/s10522-013-9443-6.
Waller KL, Mortensen EL, Avlund K, Fagerlund B, Lauritzen M, Gammeltoft S, Jennum P (2016) Melatonin and cortisol profiles in late midlife and their association with age-related changes in cognition. Nat. Sci. Sleep 8: 47-53. doi: 10.2147/NSS.S75946.
Venegas C, Garcia JA, Escames G, Ortiz F, Lopez A, Doerrier C, Garcia-Corzo L, Lopez LC, Reiter RJ, Acuna-Castroviejo D (2012) Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 52: 217-227. doi: 10.1111/j.1600-079X.2011.00931.x.
Iggena D, Winter Y, Steiner B (2017) Melatonin restores hippocampal neural precursor cell proliferation and prevents cognitive deficits induced by jet lag simulation in adult mice. J. Pineal Res. 62: e12397. doi: 10.1111/jpi.12397.
Lopez-Armas G, Flores-Soto ME, Chaparro-Huerta V, Jave-Suarez LF, Soto-Rodriguez S, Rusanova I, Acuna-Castroviejo D, Gonzalez-Perez O, Gonzalez-Castaneda RE (2016) Prophylactic role of oral melatonin administration on neurogenesis in adult Balb/C mice during REM sleep deprivation. Oxid. Med. Cell. Longev. 2016: 2136902. doi: 10.1155/2016/2136902.
Lopez-Virgen V, Zarate-Lopez D, Adirsch FL, Collas-Aguilar J, Gonzalez-Perez O (2015) Effects of sleep deprivation in hippocampal neurogenesis. Gac. Med. Mex. 151: 99-104.
Kuhn HG, Biebl M, Wilhelm D, Li M, Friedlander RM, Winkler J (2005) Increased generation of granule cells in adult Bcl-2-overexpressing mice: a role for cell death during continued hippocampal neurogenesis. Eur. J. Neurosci. 22: 1907-1915. doi: 10.1111/j.1460-9568.2005.04377.x.
Hinojosa-Godinez A, Jave-Suarez LF, Flores-Soto M, Galvez-Contreras AY, Luquin S, Oregon-Romero E, Gonzalez-Perez O, Gonzalez-Castaneda RE (2019) Melatonin modifies SOX2(+) cell proliferation in dentate gyrus and modulates SIRT1 and MECP2 in long-term sleep deprivation. Neural Regener. Res. 14: 1787-1795. doi: 10.4103/1673-5374.257537.
Gao C, Wang Q, Chung SK, Shen J (2017) Crosstalk of metabolic factors and neurogenic signaling in adult neurogenesis: Implication of metabolic regulation for mental and neurological diseases. Neurochem. Int. 106: 24-36. doi: 10.1016/j.neuint.2017.02.001.
Ziegler AN, Levison SW, Wood TL (2015) Insulin and IGF receptor signalling in neural-stem-cell homeostasis. Nat. Rev. Endocrinol. 11: 161-170. doi: 10.1038/nrendo.2014.208.
Sun P, Hua Q, Schmitt AG (2016) Energy metabolism, adult neurogenesis and their possible roles in Alzheimer's disease: a brief overview. Curr. Top. Med. Chem. 16: 493-502. doi: 10.2174/1568026615666150813142611.
Dorsemans AC, Couret D, Hoarau A, Meilhac O, Lefebvre d'Hellencourt C, Diotel N (2017) Diabetes, adult neurogenesis and brain remodeling: New insights from rodent and zebrafish models. Neurogenesis (Austin) 4: e1281862. doi: 10.1080/23262133.2017.1281862.
Velazquez R, Tran A, Ishimwe E, Denner L, Dave N, Oddo S, Dineley KT (2017) Central insulin dysregulation and energy dyshomeostasis in two mouse models of Alzheimer's disease. Neurobiol. Aging 58: 1-13. doi: 10.1016/j.neurobiolaging.2017.06.003.
Frazier HN, Ghoweri AO, Anderson KL, Lin RL, Porter NM, Thibault O (2019) Broadening the definition of brain insulin resistance in aging and Alzheimer's disease. Exp. Neurol. 313: 79-87. doi: 10.1016/j.expneurol.2018.12.007.
Gurel-Gokmen B, Ipekci H, Oktay S, Alev B, Ustundag UV, Ak E, Akakin D, Sener G, Emekli-Alturfan E, Yarat A, Tunali-Akbay T (2018) Melatonin improves hyperglycemia induced damages in rat brain. Diabetes Metab. Res. Rev. 34: e3060. doi: 10.1002/dmrr.3060.
Owino S, Contreras-Alcantara S, Baba K, Tosini G (2016) Melatonin signaling controls the daily rhythm in blood glucose levels independent of peripheral clocks. PLoS One 11: e0148214. doi: 10.1371/journal.pone.0148214.
Owino S, Buonfiglio DDC, Tchio C, Tosini G (2019) Melatonin signaling a key regulator of glucose homeostasis and energy metabolism. Front. Endocrinol. (Lausanne) 10: 488. doi: 10.3389/fendo.2019.00488.
Tiong YL, Ng KY, Koh RY, Ponnudurai G, Chye SM (2019) Melatonin prevents oxidative stress-induced mitochondrial dysfunction and apoptosis in high glucose-treated schwann cells via upregulation of Bcl2, NF-kappaB, mTOR, Wnt signalling pathways. Antioxidants (Basel) 8: 198. doi: 10.3390/antiox8070198.
Xu J, Gao H, Zhang L, Rong S, Yang W, Ma C, Chen M, Huang Q, Deng Q, Huang F (2019) Melatonin alleviates cognition impairment by antagonizing brain insulin resistance in aged rats fed a high-fat diet. J. Pineal Res. 67: e12584. doi: 10.1111/jpi.12584.
Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spégel P, Bugliani M, Saxena R, Fex M, Pulizzi N, Isomaa B, Tuomi T, Nilsson P, Kuusisto J, Tuomilehto J, Boehnke M, Altshuler D, Sundler F, Eriksson JG, Jackson AU, Laakso M, Marchetti P, Watanabe RM, Mulder H, Groop L (2009) Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat. Genet. 41: 82-88. doi:10.1038/ng.288.
McMullan CJ, Curhan GC, Schernhammer ES, Forman JP (2013) Association of nocturnal melatonin secretion with insulin resistance in nondiabetic young women. Am. J. Epidemiol. 178: 231-238. doi:10.1093/aje/kws470.
Rubio-Sastre P, Scheer FA, Gómez-Abellán P, Madrid JA, Garaulet M (2014) Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep 37: 1715-1719. doi: 10.5665/sleep.4088
Jia G, Gao Y, Li C, Zhang Y (2020) Effects of MTNR1B genetic variants on individual susceptibility to gestational diabetes mellitus: A meta-analysis. Am. J. Perinatol. 37: 607-612. doi: 10.1055/s-0039-1685446.
Tuomi T, Nagorny CLF, Singh P, Bennet H, Yu Q, Alenkvist I, Isomaa B, Östman B, Söderström J, Pesonen AK, Martikainen S, Räikkönen K, Forsén T, Hakaste L, Almgren P, Storm P, Asplund O, Shcherbina L, Fex M, Fadista J, Tengholm A, Wierup N, Groop L, Mulder H (2016) Increased melatonin signaling is a risk factor for type 2 diabetes. Cell Metab. 23: 1067-1077. doi: 10.1016/j.cmet.2016.04.009.
Li H, Zhang Y, Liu S, Li F, Wang B, Wang J, Cao L, Xia T, Yao Q, Chen H, Zhang Y, Zhu X, Li Y, Li G, Wang J, Li X, Ni S (2019) Melatonin enhances proliferation and modulates differentiation of neural stem cells via autophagy in hyperglycemia. Stem Cells 37: 504-515. doi: 10.1002/stem.2968.
Kadry SM, El-Dakdoky MH, Haggag NZ, Rashed LA, Hassen MT (2018) Melatonin improves the therapeutic role of mesenchymal stem cells in diabetic rats. Toxicol. Mech. Methods 28: 529-538. doi: 10.1080/15376516.2018.1471634.
Metwally MMM, Ebraheim LLM, Galal AAA (2018) Potential therapeutic role of melatonin on STZ-induced diabetic central neuropathy: A biochemical, histopathological, immunohistochemical and ultrastructural study. Acta Histochem. 120: 828-836. doi: 10.1016/j.acthis.2018.09.008.
Liu S, Guo Y, Yuan Q, Pan Y, Wang L, Liu Q, Wang F, Wang J, Hao A (2015) Melatonin prevents neural tube defects in the offspring of diabetic pregnancy. J. Pineal Res. 59: 508-517. doi: 10.1111/jpi.12282.
Gao Y, Bai C, Zheng D, Li C, Zhang W, Li M, Guan W, Ma Y (2016) Combination of melatonin and Wnt-4 promotes neural cell differentiation in bovine amniotic epithelial cells and recovery from spinal cord injury. J. Pineal Res. 60: 303-312. doi: 10.1111/jpi.12311.
Yoo DY, Kim W, Lee CH, Shin BN, Nam SM, Choi JH, Won MH, Yoon YS, Hwang IK (2012) Melatonin improves D-galactose-induced aging effects on behavior, neurogenesis, and lipid peroxidation in the mouse dentate gyrus via increasing pCREB expression. J. Pineal Res. 52: 21-28. doi: 10.1111/j.1600-079X.2011.00912.x.
Wongchitrat P, Lansubsakul N, Kamsrijai U, Sae-Ung K, Mukda S, Govitrapong P (2016) Melatonin attenuates the high-fat diet and streptozotocin-induced reduction in rat hippocampal neurogenesis. Neurochem. Int. 100: 97-109. doi: 10.1016/j.neuint.2016.09.006.
Guo YL, Chakraborty S, Rajan SS, Wang R, Huang F (2010) Effects of oxidative stress on mouse embryonic stem cell proliferation, apoptosis, senescence, and self-renewal. Stem Cells Dev. 19: 1321-1331. doi: 10.1089/scd.2009.0313.
Furio AM., Fontao R, Falco N, Ruiz JI, Caccuri R, Cardinali DP.(2008) Neuroprotective Effect of Melatonin on Glucocorticoid Toxicity in the Rat Hippocampus. Open Physiol. J. 1: 23-27. doi: 10.2174/1874360900901010023.
Ruksee N, Tongjaroenbuangam W, Mahanam T, Govitrapong P (2014) Melatonin pretreatment prevented the effect of dexamethasone negative alterations on behavior and hippocampal neurogenesis in the mouse brain. J. Steroid Biochem. Mol. Biol. 143: 72-80. doi: 10.1016/j.jsbmb.2014.02.011.
Fu J, Zhao SD, Liu HJ, Yuan QH, Liu SM, Zhang YM, Ling EA, Hao AJ (2011) Melatonin promotes proliferation and differentiation of neural stem cells subjected to hypoxia in vitro. J. Pineal Res. 51: 104-112. doi: 10.1111/j.1600-079X.2011.00867.x.
Permpoonputtana K, Tangweerasing P, Mukda S, Boontem P, Nopparat C, Govitrapong P (2018) Long-term administration of melatonin attenuates neuroinflammation in the aged mouse brain. EXCLI J. 17: 634-646. doi: 10.17179/excli2017-654.
Ghareghani M, Sadeghi H, Zibara K, Danaei N, Azari H, Ghanbari A (2017) Melatonin increases oligodendrocyte differentiation in cultured neural stem cells. Cell. Mol. Neurobiol. 37: 1319-1324. doi: 10.1007/s10571-016-0450-4.
Manda K,Reiter RJ (2010) Melatonin maintains adult hippocampal neurogenesis and cognitive functions after irradiation. Prog. Neurobiol. 90: 60-68. doi: 10.1016/j.pneurobio.2009.10.019.
Chen BH, Park JH, Lee TK, Song M, Kim H, Lee JC, Kim YM, Lee CH, Hwang IK, Kang IJ, Yan BC, Won MH, Ahn JH (2018) Melatonin attenuates scopolamine-induced cognitive impairment via protecting against demyelination through BDNF-TrkB signaling in the mouse dentate gyrus. Chem. Biol. Interact. 285: 8-13. doi: 10.1016/j.cbi.2018.02.023.
Aranarochana A, Chaisawang P, Sirichoat A, Pannangrong W, Wigmore P, Welbat JU (2019) Protective effects of melatonin against valproic acid-induced memory impairments and reductions in adult rat hippocampal neurogenesis. Neuroscience 406: 580-593. doi: 10.1016/j.neuroscience.2019.02.022.
Sirichoat A, Krutsri S, Suwannakot K, Aranarochana A, Chaisawang P, Pannangrong W, Wigmore P, Welbat JU (2019) Melatonin protects against methotrexate-induced memory deficit and hippocampal neurogenesis impairment in a rat model. Biochem. Pharmacol. 163: 225-233. doi: 10.1016/j.bcp.2019.02.010.
Han J, Ji C, Guo Y, Yan R, Hong T, Dou Y, An Y, Tao S, Qin F, Nie J, Ji C, Wang H, Tong J, Xiao W, Zhang J (2017) Mechanisms underlying melatonin-mediated prevention of fenvalerate-induced behavioral and oxidative toxicity in zebrafish. J. Toxicol. Environ. Health Part A 80: 1331-1341. doi: 10.1080/15287394.2017.1384167.
Garcia-Cabrerizo R,Garcia-Fuster MJ (2016) Comparative effects of amphetamine-like psychostimulants on rat hippocampal cell genesis at different developmental ages. Neurotoxicology 56: 29-39. doi: 10.1016/j.neuro.2016.06.014.
Takashima Y,Mandyam CD (2018) The role of hippocampal adult neurogenesis in methamphetamine addiction. Brain Plast. 3: 157-168. doi: 10.3233/BPL-170058.
Dutta RR, Taffe MA, Mandyam CD (2018) Chronic administration of amphetamines disturbs development of neural progenitor cells in young adult nonhuman primates. Prog. Neuropsychopharmacol. Biol. Psychiatry 85: 46-53. doi: 10.1016/j.pnpbp.2018.03.023.
Singhakumar R, Boontem P, Ekthuwapranee K, Sotthibundhu A, Mukda S, Chetsawang B, Govitrapong P (2015) Melatonin attenuates methamphetamine-induced inhibition of neurogenesis in the adult mouse hippocampus: An in vivo study. Neurosci. Lett. 606: 209-214. doi: 10.1016/j.neulet.2015.09.011.
Ekthuwapranee K, Sotthibundhu A, Govitrapong P (2015) Melatonin attenuates methamphetamine-induced inhibition of proliferation of adult rat hippocampal progenitor cells in vitro. J. Pineal Res. 58: 418-428. doi: 10.1111/jpi.12225.
Ekthuwapranee K, Sotthibundhu A, Tocharus C, Govitrapong P (2015) Melatonin ameliorates dexamethasone-induced inhibitory effects on the proliferation of cultured progenitor cells obtained from adult rat hippocampus. J. Steroid Biochem. Mol. Biol. 145: 38-48. doi: 10.1016/j.jsbmb.2014.10.003.
Dorostkar MM, Zou C, Blazquez-Llorca L, Herms J (2015) Analyzing dendritic spine pathology in Alzheimer's disease: problems and opportunities. Acta Neuropathol. 130: 1-19. doi: 10.1007/s00401-015-1449-5.
Dominguez-Alonso A, Valdes-Tovar M, Solis-Chagoyan H, Benitez-King G (2015) Melatonin stimulates dendrite formation and complexity in the hilar zone of the rat hippocampus: participation of the Ca++/Calmodulin complex. Int. J. Mol. Sci. 16: 1907-1927. doi: 10.3390/ijms16011907.
Hill AS, Sahay A, Hen R (2015) Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression-like behaviors. Neuropsychopharmacology 40: 2368-2378. doi: 10.1038/npp.2015.85.
Oglodek EA, Just MJ, Szromek AR, Araszkiewicz A (2016) Melatonin and neurotrophins NT-3, BDNF, NGF in patients with varying levels of depression severity. Pharmacol. Rep. 68: 945-951. doi: 10.1016/j.pharep.2016.04.003.
Haridas S, Kumar M, Manda K (2012) Chronic melatonin administration mitigates behavioral dysfunction induced by gamma-irradiation. Horm. Behav. 62: 621-627. doi: 10.1016/j.yhbeh.2012.09.006.
Lee Y, Lee S, Lee SR, Park K, Hong Y, Lee M, Park S, Jin Y, Chang KT, Hong Y (2014) Beneficial effects of melatonin combined with exercise on endogenous neural stem/progenitor cells proliferation after spinal cord injury. Int. J. Mol. Sci. 15: 2207-2222. doi: 10.3390/ijms15022207.
Liu J, Somera-Molina KC, Hudson RL, Dubocovich ML (2013) Melatonin potentiates running wheel-induced neurogenesis in the dentate gyrus of adult C3H/HeN mice hippocampus. J. Pineal Res. 54: 222-231. doi: 10.1111/jpi.12023.
Spires-Jones TL,Ritchie CW (2018) A brain boost to fight Alzheimer's disease. Science 361: 975-976. doi: 10.1126/science.aau8060.
Michalski D, Hofmann S, Pitsch R, Grosche J, Hartig W (2017) Neurovascular specifications in the Alzheimer-like brain of mice affected by focal cerebral ischemia: implications for future therapies. J. Alzheimers Dis. 59: 655-674. doi: 10.3233/JAD-170185.
Tang Y, Cai B, Yuan F, He X, Lin X, Wang J, Wang Y, Yang GY (2014) Melatonin pretreatment improves the survival and function of transplanted mesenchymal stem cells after focal cerebral ischemia. Cell Transplant. 23: 1279-1291. doi: 10.3727/096368913x667510.
Lin L, Huang QX, Yang SS, Chu J, Wang JZ, Tian Q (2013) Melatonin in Alzheimer's disease. Int. J. Mol. Sci. 14: 14575-14593. doi: 10.3390/ijms140714575.
Tang YP, Ma YL, Chao CC, Chen KY, Lee EH (1998) Enhanced glial cell line-derived neurotrophic factor mRNA expression upon (-)-deprenyl and melatonin treatments. J. Neurosci. Res. 53: 593-604. doi: 10.1002/(SICI)1097-4547(19980901)53:5<593::AID-JNR9>3.0.CO;2-4.
Borlongan CV, Yamamoto M, Takei N, Kumazaki M, Ungsuparkorn C, Hida H, Sanberg PR, Nishino H (2000) Glial cell survival is enhanced during melatonin-induced neuroprotection against cerebral ischemia. FASEB J. 14: 1307-1317. doi: 10.1096/fj.14.10.1307.
Heneka MT, Carson MJ, Khoury JE, Landreth GE, Brosseron F, Feinstein DL , Jacobs AH , Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC , Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA , Breitner JC, Cole GM, Golenbock DT, Kummer MP (2015) Neuroinflammation in Alzheimer's disease. Lancet Neurol. 14: 388‐405. doi:10.1016/S1474-4422(15)70016-5.
Kalamakis G, Brüne D, Ravichandran S, Bolz J, Fan W, Ziebell F, Stiehl T, Catalá-Martinez F, Kupke J, Zhao S, Llorens-Bobadilla E, Bauer K Limpert S, Berger B, Christen U, Schmezer P, Mallm JP, Berninger B, Anders S, Sol AD, Marciniak-Czochra A, Martin-Villalba A (2019) Quiescence modulates stem cell maintenance and regenerative capacity in the aging brain. Cell 176: 1407-1419.e14. doi: 10.1016/j.cell.2019.01.040.
E L, Burns JM, Swerdlow RH. (2014) Effect of high-intensity exercise on aged mouse brain mitochondria, neurogenesis, and inflammation. Neurobiol. Aging 35: 2574‐2583. doi: 10.1016/j.neurobiolaging.05.033
Valero J, Bernardino L, Cardoso FL, Silva AP, Fontes-Ribeiro C, Ambrósio AF, Malva JO (2017) Impact of neuroinflammation on hippocampal neurogenesis: relevance to aging and Alzheimer's disease. J. Alzheimers Dis. 60: S161-S168. doi: 10.3233/JAD-170239
Kohman RA, Rhodes JS (2013) Neurogenesis, inflammation and behavior. Brain Behav. Immun. 27: 22-32. doi: 10.1016/j.bbi.2012.09.003
Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J (2015) Microglial and macrophage polarization—new prospects for brain repair. Nat. Rev. Neurol. 11: 56-64. doi: 10.1038/nrneurol.2014.207.
Xia Y, Chen S, Zeng S, Zhao Y, Zhu C, Deng B, Zhu G, Yin Y, Wang W, Hardeland R, Ren W (2019) Melatonin in macrophage biology: Current understanding and future perspectives. J. Pineal Res. 66: e12547. doi: 10.1111/jpi.12547.
Chinchalongporn V, Shukla M, Govitrapong P (2018) Melatonin ameliorates Abeta42 -induced alteration of betaAPP-processing secretases via the melatonin receptor through the Pin1/GSK3beta/NF-kappaB pathway in SH-SY5Y cells. J. Pineal Res. 64: e12470. doi: 10.1111/jpi.12470.
de la Fuente Revenga M, Fernandez-Saez N, Herrera-Arozamena C, Morales-Garcia JA, Alonso-Gil S, Perez-Castillo A, Caignard DH, Rivara S, Rodriguez-Franco MI (2015) Novel N-acetyl bioisosteres of melatonin: melatonergic receptor pharmacology, physicochemical studies, and phenotypic assessment of their neurogenic potential. J. Med. Chem. 58: 4998-5014. doi: 10.1021/acs.jmedchem.5b00245.
Sompol P, Liu X, Baba K, Paul KN, Tosini G, Iuvone PM, Ye K (2011) N-acetylserotonin promotes hippocampal neuroprogenitor cell proliferation in sleep-deprived mice. Proc. Natl. Acad. Sci. 108: 8844-8849. doi: 10.1073/pnas.1105114108.
Tosini G, Baba K, Hwang CK, Iuvone PM (2012) Melatonin: an underappreciated player in retinal physiology and pathophysiology. Exp. Eye Res. 103: 82-89. doi: 10.1016/j.exer.2012.08.009.
Manda K, Ueno M, Anzai K (2008) Space radiation-induced inhibition of neurogenesis in the hippocampal dentate gyrus and memory impairment in mice: ameliorative potential of the melatonin metabolite, AFMK. J. Pineal Res. 45: 430-438. doi: 10.1111/j.1600-079X.2008.00611.x.
Manda K, Ueno M, Anzai K (2009) Cranial irradiation-induced inhibition of neurogenesis in hippocampal dentate gyrus of adult mice: attenuation by melatonin pretreatment. J. Pineal Res. 46: 71-78. doi: 10.1111/j.1600-079X.2008.00632.x.
Soumier A, Banasr M, Lortet S, Masmejean F, Bernard N, Kerkerian-Le-Goff L, Gabriel C, Millan MJ, Mocaer E, Daszuta A (2009) Mechanisms contributing to the phase-dependent regulation of neurogenesis by the novel antidepressant, agomelatine, in the adult rat hippocampus. Neuropsychopharmacology 34: 2390-2403. doi: 10.1038/npp.2009.72.
Yucel A, Yucel N, Ozkanlar S, Polat E, Kara A, Ozcan H, Gulec M (2016) Effect of agomelatine on adult hippocampus apoptosis and neurogenesis using the stress model of rats. Acta Histochem. 118: 299-304. doi: 10.1016/j.acthis.2016.02.007.
Fu W, Xie H, Laudon M, Zhou S, Tian S, You Y (2016) Piromelatine ameliorates memory deficits associated with chronic mild stress-induced anhedonia in rats. Psychopharmacology (Berl.) 233: 2229-2239. doi: 10.1007/s00213-016-4272-3.
Figueiro-Silva J, Antequera D, Pascual C, de la Fuente Revenga M, Volt H, Acuna-Castroviejo D, Rodriguez-Franco MI, Carro E (2018) The melatonin analog IQM316 may induce adult hippocampal neurogenesis and preserve recognition memories in mice. Cell Transplant. 27: 423-437. doi: 10.1177/0963689717721217.
Thompson PM, Hayashi KM, Dutton RA, Chiang MC, Leow AD, Sowell ER, De Zubicaray G, Becker JT, Lopez OL, Aizenstein HJ, Toga AW (2007) Tracking Alzheimer's disease. Ann. N. Y. Acad. Sci. 1097: 183-214. doi: 10.1196/annals.1379.017.
Taupin P (2006) Neurogenesis and Alzheimer's disease. Drug Target Insights 1: 1-4.
Leyssen M, Ayaz D, Hebert SS, Reeve S, De Strooper B, Hassan BA (2005) Amyloid precursor protein promotes post-developmental neurite arborization in the Drosophila brain. EMBO J. 24: 2944-2955. doi: 10.1038/sj.emboj.7600757.
Hornsten A, Lieberthal J, Fadia S, Malins R, Ha L, Xu X, Daigle I, Markowitz M, O'Connor G, Plasterk R, Li C (2007) APL-1, a Caenorhabditis elegans protein related to the human beta-amyloid precursor protein, is essential for viability. Proc. Natl. Acad. Sci. 104: 1971-1976. doi: 10.1073/pnas.0603997104.
Hung AY, Koo EH, Haass C, Selkoe DJ (1992) Increased expression of beta-amyloid precursor protein during neuronal differentiation is not accompanied by secretory cleavage. Proc. Natl. Acad. Sci. 89: 9439-9443. doi: 10.1073/pnas.89.20.9439.
Salbaum JM,Ruddle FH (1994) Embryonic expression pattern of amyloid protein precursor suggests a role in differentiation of specific subsets of neurons. J. Exp. Zool. 269: 116-127. doi: 10.1002/jez.1402690205.
Feng R, Rampon C, Tang YP, Shrom D, Jin J, Kyin M, Sopher B, Miller MW, Ware CB, Martin GM, Kim SH, Langdon RB, Sisodia SS, Tsien JZ (2001) Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron 32: 911-926. doi: 10.1016/s0896-6273(01)00523-2.
Wen PH, Shao X, Shao Z, Hof PR, Wisniewski T, Kelley K, Friedrich VL, Jr., Ho L, Pasinetti GM, Shioi J, Robakis NK, Elder GA (2002) Overexpression of wild type but not an FAD mutant presenilin-1 promotes neurogenesis in the hippocampus of adult mice. Neurobiol. Dis. 10: 8-19. doi: 10.1006/nbdi.2002.0490.
Donovan MH, Yazdani U, Norris RD, Games D, German DC, Eisch AJ (2006) Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer's disease. J. Comp. Neurol. 495: 70-83. doi: 10.1002/cne.20840.
Zhang C, McNeil E, Dressler L, Siman R (2007) Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer's disease. Exp. Neurol. 204: 77-87. doi: 10.1016/j.expneurol.2006.09.018.
Zhou JN, Liu RY, Kamphorst W, Hofman MA, Swaab DF (2003) Early neuropathological Alzheimer's changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. J. Pineal Res. 35: 125-130. doi: 10.1034/j.1600-079x.2003.00065.x.
Komuro Y, Xu G, Bhaskar K, Lamb BT (2015) Human tau expression reduces adult neurogenesis in a mouse model of tauopathy. Neurobiol. Aging 36: 2034-2042. doi: 10.1016/j.neurobiolaging.2015.03.002.
Ali T,Kim MO (2015) Melatonin ameliorates amyloid beta-induced memory deficits, tau hyperphosphorylation and neurodegeneration via PI3/Akt/GSk3beta pathway in the mouse hippocampus. J. Pineal Res. 59: 47-59. doi: 10.1111/jpi.12238.
Deng YQ, Xu GG, Duan P, Zhang Q, Wang JZ (2005) Effects of melatonin on wortmannin-induced tau hyperphosphorylation. Acta Pharmacol. Sin. 26: 519-526. doi: 10.1111/j.1745-7254.2005.00102.x.
Pappolla MA, Sos M, Omar RA, Bick RJ, Hickson-Bick DL, Reiter RJ, Efthimiopoulos S, Robakis NK (1997) Melatonin prevents death of neuroblastoma cells exposed to the Alzheimer amyloid peptide. J. Neurosci. 17: 1683-1690.
Pappolla MA, Chyan YJ, Poeggeler B, Frangione B, Wilson G, Ghiso J, Reiter RJ (2000) An assessment of the antioxidant and the antiamyloidogenic properties of melatonin: implications for Alzheimer's disease. J. Neural Transm. 107: 203-231. doi: 10.1007/s007020050018.
Olcese JM, Cao C, Mori T, Mamcarz MB, Maxwell A, Runfeldt MJ, Wang L, Zhang C, Lin X, Zhang G, Arendash GW (2009) Protection against cognitive deficits and markers of neurodegeneration by long-term oral administration of melatonin in a transgenic model of Alzheimer disease. J. Pineal Res. 47: 82-96. doi: 10.1111/j.1600-079X.2009.00692.x.
Suh J, Choi SH, Romano DM, Gannon MA, Lesinski AN, Kim DY, Tanzi RE (2013) ADAM10 missense mutations potentiate beta-amyloid accumulation by impairing prodomain chaperone function. Neuron 80: 385-401. doi: 10.1016/j.neuron.2013.08.035.
Ryan MM, Morris GP, Mockett BG, Bourne K, Abraham WC, Tate WP, Williams JM (2013) Time-dependent changes in gene expression induced by secreted amyloid precursor protein-alpha in the rat hippocampus. BMC Genomics 14: 376. doi: 10.1186/1471-2164-14-376.
Shukla M, Htoo HH, Wintachai P, Hernandez JF, Dubois C, Postina R, Xu H, Checler F, Smith DR, Govitrapong P, Vincent B (2015) Melatonin stimulates the nonamyloidogenic processing of betaAPP through the positive transcriptional regulation of ADAM10 and ADAM17. J. Pineal Res. 58: 151-165. doi: 10.1111/jpi.12200.
Endres K,Deller T (2017) Regulation of alpha-secretase ADAM10 in vitro and in vivo: genetic, epigenetic, and protein-based mechanisms. Front. Mol. Neurosci. 10: 56. doi: 10.3389/fnmol.2017.00056.
Habib A, Sawmiller D, Tan J (2017) Restoring soluble amyloid precursor protein alpha functions as a potential treatment for Alzheimer's disease. J. Neurosci. Res. 95: 973-991. doi: 10.1002/jnr.23823.
Colciaghi F, Borroni B, Pastorino L, Marcello E, Zimmermann M, Cattabeni F, Padovani A, Di Luca M (2002) [alpha]-Secretase ADAM10 as well as [alpha]APPs is reduced in platelets and CSF of Alzheimer disease patients. Mol. Med. 8: 67-74.
Caille I, Allinquant B, Dupont E, Bouillot C, Langer A, Muller U, Prochiantz A (2004) Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development 131: 2173-2181. doi: 10.1242/dev.01103.
Demars MP, Bartholomew A, Strakova Z, Lazarov O (2011) Soluble amyloid precursor protein: a novel proliferation factor of adult progenitor cells of ectodermal and mesodermal origin. Stem Cell. Res. Ther. 2: 36. doi: 10.1186/scrt77.
Lazarov O,Demars MP (2012) All in the family: How the APPs regulate neurogenesis. Front. Neurosci. 6: 81. doi: 10.3389/fnins.2012.00081.
Mattson MP (1997) Cellular actions of beta-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol. Rev. 77: 1081-1132.
Gakhar-Koppole N, Hundeshagen P, Mandl C, Weyer SW, Allinquant B, Muller U, Ciccolini F (2008) Activity requires soluble amyloid precursor protein alpha to promote neurite outgrowth in neural stem cell-derived neurons via activation of the MAPK pathway. Eur. J. Neurosci. 28: 871-882. doi: 10.1111/j.1460-9568.2008.06398.x.
Ohsawa I, Takamura C, Morimoto T, Ishiguro M, Kohsaka S (1999) Amino-terminal region of secreted form of amyloid precursor protein stimulates proliferation of neural stem cells. Eur. J. Neurosci. 11: 1907-1913. doi: 10.1046/j.1460-9568.1999.00601.x.
Bell KF, Zheng L, Fahrenholz F, Cuello AC (2008) ADAM-10 over-expression increases cortical synaptogenesis. Neurobiol. Aging 29: 554-565. doi: 10.1016/j.neurobiolaging.2006.11.004.
Han P, Dou F, Li F, Zhang X, Zhang YW, Zheng H, Lipton SA, Xu H, Liao FF (2005) Suppression of cyclin-dependent kinase 5 activation by amyloid precursor protein: a novel excitoprotective mechanism involving modulation of tau phosphorylation. J. Neurosci. 25: 11542-11552. doi: 10.1523/JNEUROSCI.3831-05.2005.
Curtis MA, Faull RL, Eriksson PS (2007) The effect of neurodegenerative diseases on the subventricular zone. Nat. Rev. Neurosci. 8: 712-723. doi: 10.1038/nrn2216.
Ayuso-Sacido A, Moliterno JA, Kratovac S, Kapoor GS, O'Rourke DM, Holland EC, Garcia-Verdugo JM, Roy NS, Boockvar JA (2010) Activated EGFR signaling increases proliferation, survival, and migration and blocks neuronal differentiation in post-natal neural stem cells. J. Neurooncol. 97: 323-337. doi: 10.1007/s11060-009-0035-x.
Chen Q, Nakajima A, Choi SH, Xiong X, Sisodia SS, Tang YP (2008) Adult neurogenesis is functionally associated with AD-like neurodegeneration. Neurobiol. Dis. 29: 316-326. doi: 10.1016/j.nbd.2007.09.005.
Ghosal K, Stathopoulos A, Pimplikar SW (2010) APP intracellular domain impairs adult neurogenesis in transgenic mice by inducing neuroinflammation. PLoS One 5: e11866. doi: 10.1371/journal.pone.0011866.
Sarlak G, Htoo HH, Hernandez JF, Iizasa H, Checler F, Konietzko U, Song W, Vincent B (2016) Sox2 functionally interacts with betaAPP, the betaAPP intracellular domain and ADAM10 at a transcriptional level in human cells. Neuroscience 312: 153-164. doi: 10.1016/j.neuroscience.2015.11.022.
Sarlak G,Vincent B (2016) The roles of the stem cell-controlling Sox2 transcription factor: from neuroectoderm development to Alzheimer's disease? Mol. Neurobiol. 53: 1679-1698. doi: 10.1007/s12035-015-9123-4.
Wei R, Zhao X, Hao H, Du W, Zhu H (2016) Embryonic stem-like cells from rabbit blastocysts cultured with melatonin could differentiate into three germ layers in vitro and in vivo. Mol. Reprod. Dev. 83: 1003-1014. doi: 10.1002/mrd.22739.
Hu X, Das B, Hou H, He W, Yan R (2018) BACE1 deletion in the adult mouse reverses preformed amyloid deposition and improves cognitive functions. J. Exp. Med. 215: 927-940. doi: 10.1084/jem.20171831.
Chatila ZK, Kim E, Berle C, Bylykbashi E, Rompala A, Oram MK, Gupta D, Kwak SS, Kim YH, Kim DY, Choi SH, Tanzi RE (2018) BACE1 regulates proliferation and neuronal differentiation of newborn cells in the adult hippocampus in mice. eNeuro 5: doi: 10.1523/ENEURO.0067-18.2018.
Gadadhar A, Marr R, Lazarov O (2011) Presenilin-1 regulates neural progenitor cell differentiation in the adult brain. J. Neurosci. 31: 2615-2623. doi: 10.1523/JNEUROSCI.4767-10.2011.
Bonds JA, Kuttner-Hirshler Y, Bartolotti N, Tobin MK, Pizzi M, Marr R, Lazarov O (2015) Presenilin-1 dependent neurogenesis regulates hippocampal learning and memory. PLoS One 10: e0131266. doi: 10.1371/journal.pone.0131266.
Panmanee J, Nopparat C, Chavanich N, Shukla M, Mukda S, Song W, Vincent B, Govitrapong P (2015) Melatonin regulates the transcription of betaAPP-cleaving secretases mediated through melatonin receptors in human neuroblastoma SH-SY5Y cells. J. Pineal Res. 59: 308-320. doi: 10.1111/jpi.12260.
Mukda S, Panmanee J, Boontem P, Govitrapong P (2016) Melatonin administration reverses the alteration of amyloid precursor protein-cleaving secretases expression in aged mouse hippocampus. Neurosci. Lett. 621: 39-46. doi: 10.1016/j.neulet.2016.04.013.
Karasek K., Winczyk K (2006) Melatonin in humans. J. Physiol. Pharmacol. 57: 19–39.
Di WL, Kadva A, Johnston A, Silman R (1997) Variable bioavailability of oral melatonin. N. Engl. J. Med. 336: 1028–1029. doi: 10.1056/NEJM199704033361418.
Brusco LI, Márquez M, Cardinali DP (2000) Melatonin treatment stabilizes chronobiologic and cognitive symptoms in Alzheimer's disease. Neuro. Endocrinol. Lett. 21: 39‐42.
Kilic E, Kilic U, Bacigaluppi M, Guo Z, Ben Abdallah N, Wolfer DP, Reiter RJ, Hermann DM, Bassetti CL (2008) Delayed melatonin administration promotes neuronal survival, neurogenesis and motor recovery, and attenuates hyperactivity and anxiety after mild focal cerebral ischemia in mice. J. Pineal Res. 45: 142–148. doi: 10.1111/j.1600-079X.2008.00568.x.
López-Armas G, Flores-Soto ME, Chaparro-Huerta V, Jave-Suarez LF, Soto-Rodríguez S, Rusanova I, Acuña-Castroviejo D, González-Perez O, González-Castañeda RE (2016) Prophylactic role of oral melatonin administration on neurogenesis in adult Balb/c mice during rem sleep deprivation. Oxid. Med. Cell Longev. 2016:2136902. doi:10.1155/2016/2136902.
Ramírez-Rodríguez G, Klempin F, Babu H. Benítez-King G, Kempermann G (2009) Melatonin modulates cell survival of new neurons in the hippocampus of adult mice. Neuropsychopharmacol. 34: 2180–2191. doi: 10.1038/npp.2009.46.
Zhdanova IV, Geiger DA, Schwagerl AL, Leclair OU, Killiany R, Taylor JA, Rosene DL, Moss MB, Madras BK (2002) Melatonin promotes sleep in three species of diurnal nonhuman primates. Physiol. Behav. 75: 523-529. doi: 10.1016/s0031-9384(02)00654-6.
Polimeni G, Esposito E, Bevelacqua V, Guarneri C, Cuzzocrea S (2014) Role of melatonin supplementation in neurodegenerative disorders. Front. Biosci. (Landmark Ed.) 19: 429–446. doi: 10.2741/4217.
Tordjman S, Chokron S, Delorme R, Charrier A, Bellissant E, Jaafari N , Fougerou (2017) Melatonin: pharmacology, functions and therapeutic benefits. Curr. Neuropharmacol. 15: 434‐443. doi:10.2174/1570159X14666161228122115.

This work is licensed under a Creative Commons Attribution 4.0 International License